Freedom and security: “there is no cure for asthma”
Children attending the first Australian specialist paediatric asthma clinic, established in 1960, were told: “There is no cure for asthma. Freedom and security from attacks will depend on how efficiently treatment is carried out” (Box 1).1 At this time, therapy was limited, focused on relieving symptoms and preventing acute and life‐threatening airway obstruction. Early drugs had no long term therapeutic benefit, and hopes that inhaled corticosteroids might modify the course of the disease were not fulfilled. With the introduction of combination long acting reliever and controller therapies and, later, of monoclonal antibody biologic agents, we have seen significant advances in asthma management, opening the possibility of achieving on‐treatment remission in some people with asthma, typically those with less severe disease and of shorter duration. Although the efficacy of current approaches for providing lasting off‐treatment remission remains to be tested, people with asthma have reported sustained remission, and that a cure is desirable. Interviews with adults with asthma in 2003 found that 36% expected an asthma cure; this expectation was considered unrealistic by researchers,2 their perspectives reflecting what could be achieved with the best care.
In this article, we challenge the stagnant mindset that expectations of asthma cures are unrealistic, providing evidence that new technologies and fundamental discoveries will provide inflection points for accelerating progress to a cure. Recent evidence for on‐treatment remission has invigorated researchers to consider novel approaches to correct the underlying pathology of asthma by reversing bronchial hyperreactivity, pathological remodelling, and mucus plugging. We must fundamentally direct drug discovery and treatment paradigms to finding cures.
Moving beyond inflammation
Asthma was once considered a singular disease of bronchoconstriction, and then a chronic inflammatory disease.3 Corticosteroids transformed therapy for many, but not for the small but substantial minority of people with severe disease. Further, systemic corticosteroids are associated with toxic adverse effects at doses as low as 1000 mg over a lifetime.4 Most importantly, corticosteroids do not cure asthma, and there is little convincing evidence that they modify the disease trajectories of people with progressive disease. Severe asthma is characterised by the lack of or only refractory response to inhaled corticosteroids for controlling airway inflammation. Paradoxically, in response to the indisputable need to reduce the high cost and burden of severe asthma, we have inadvertently removed the emphasis from targeting its earlier and milder stages, when the chances for off‐treatment remission are possibly greater. Achieving on‐treatment remission with biologic agents is based on the now widely adopted endotype concept, that asthma includes many different forms for which the distinct underlying molecular mechanisms must be matched by targeted therapies.5 Treatment paradigms have since been expanded further to take individual susceptibility into account with a direct treatable traits approach.6 We foresee the rapid development of a treat‐to‐target approach, analogous to successful strategies in cancer and rheumatoid arthritis therapy, for which, cures and lasting off‐treatment remissions are increasingly more achievable.7 Unlike cancer or rheumatoid arthritis, however, the absence of a curative surgical procedure or a broadly efficacious disease‐modifying drug for asthma remains a problem.
A vision for asthma cures
Leading Australian clinicians, researchers, and Asthma Foundation representatives discussed a central question at the first CURE Asthma Research Symposium in April 2024: what defines a curative asthma medication? A cure paradigm (as discussed by Thomas and colleagues in this supplement8) needs to go beyond the current scope of disease modification and remission‐focused paradigms. Determining whether a medication has led to a cure should therefore be based on the absence of symptoms or signs of asthma; be induced by the treatment but be sustained after its cessation; and lead to the apparent absence of the pathophysiological features of asthma. An exemplary framework9 illustrates the multifaceted components and evolution of modern asthma treatment concepts (Box 2). Building on this framework, and considering that current treatments do not aim to cure asthma, our vision is essentially to identify distinct endotype‐specific opportunities at specific stages of asthma at which the disease process is most plastic and amenable to modification (Box 3).
A reductionist approach to an early asthma cure that could be achievable would be through the discovery of a modifiable pre‐disease intervention period. A modifiable pre‐asthma state would be most feasibly defined and validated first in children, minimising the temporal complexity of gene–environment pathogenesis hypotheses, although the lack of defined causality remains a major limitation. The most established candidate for causality is viral infection in children, particularly severe bronchiolitis, which often precedes the development of asthma. Respiratory syncytial virus (RSV) bronchiolitis is strongly associated with future asthma development,10 impaired lung function, and pre‐school age wheeze.11 Effective treatments are available for reducing the risk of severe RSV bronchiolitis. If causality can be established, evidence‐based prevention in a substantial fraction of children at risk of developing asthma with monoclonal antibodies or vaccination programs12 could lead to the discovery of a modifiable molecular pre‐asthma state. We need only to consider the profound reduction in cervical cancer incidence achieved by vaccination against the human papillomavirus to imagine that refined antiviral approaches could achieve a similar effect on the incidence of asthma, particularly in children. Understanding at the molecular level how risk factors such as atopy, infection, and poor air quality cause incident asthma would then define an intervenable therapeutic pre‐asthma window. Specifically, allergy shifts the immune system towards a type 2‐high asthma state, but when and precisely how this shift from allergy to asthma becomes established is unclear. Insights into the spatial and cellular complexity of childhood asthma, building on the new technologies discussed by Quon and colleagues in this supplement,13 could enable researchers to answer these questions.
An intervention based on a pre‐asthma paradigm would not be a cure for people with established disease, particularly those with sudden adult‐onset asthma or asthma that predisposes them to fixed airflow limitation in later life. Over time, the molecular drivers of the pathophysiology of asthma become increasingly heterogeneous and difficult to reverse, making the disease less modifiable. Indeed, recent findings indicate that anomalies in airway smooth muscle in people with asthma are highly heterogeneous, and that their persistence despite optimal anti‐inflammatory therapy suggests mechanisms independent of airway inflammation.14 Multiple therapies will therefore be needed to reverse airway remodelling; remission will be initially achieved by reducing inflammation, followed by interventions that reverse structural anomalies. Reversing airway smooth muscle remodelling has long been pursued as a strategy for modifying asthma progression. A recent response to the impending retirement of bronchial thermoplasty captured the complexity, history, and crucial lessons of the successes and failures of this intervention.15 Importantly, the lack of knowledge about how it improved asthma control led to reluctance about more broadly adopting bronchial thermoplasty, which was controversial because few systematic large randomised controlled trials were undertaken.
More fundamental insights into the less researched upstream molecular regulatory mechanisms that have gone awry in established persistent disease are central to restoring airway health in asthma. Untangling the intricate interactions of immune and airway cells in the initiation of molecular processes underlying smooth muscle proliferation could identify novel epigenetic programming or molecular pathways that could be targeted for redirecting the thickened, diseased airway to a healthy state. The failure of the respiratory epithelium to restore and repair itself in people with asthma perpetuates defects of epigenetic maturation that, in turn, lead to highly dysfunctional mucus cell lineages that respond inappropriately to viral infections and bacterial colonisation. Harnessing the self‐regenerative capacity of the airway epithelium could provide novel solutions for both reducing mucus obstruction and restoring airway health. Novel therapies that redirect cell lineages, transforming cells that would have become diseased, have shown promise for resolving mucus obstruction and repairing the airway epithelium.16 Anti‐IL‐4/13 therapy directly inhibits the effects of IL‐13‐induced mucus hypersecretion,17 while anti‐IL‐5 biologic agents reduce eosinophilic inflammation and degranulation, reducing the release of eosinophil granular proteins that directly contribute to mucus hyperviscosity and stasis.18 Our limited understanding of the nature of mucus in different forms of asthma hinders the development of mucus‐directed therapies. The composition and biochemical properties of mucus in asthma differ from those of mucus in other obstructive conditions.19 Just as targeting the specific allergy of someone with asthma is effective, the heterogeneity of mucus in different types of asthma could indicate that targeted mucus‐directed therapies for muco‐obstructive endotypes of asthma might be useful. The plethora of recently emergent molecular and cellular‐directed technologies have established a landscape in which the question is not which type of drug should be used, but rather the need to discover the fundamental molecular mechanisms that can be targeted.
Re‐thinking drug discovery and treatment paradigms for an asthma cure
Asthma research over the past century has produced precise and highly effective treatments. Sustained on‐treatment remission is now possible, as treatment paradigms shift toward precision and move away from a one‐size‐fits‐all approach. Australian asthma research has a long history of transforming asthma management and care. However, to achieve asthma cures, drug development and treatment paradigms must transition to a cure mindset. Unravelling the deep molecular architecture of disease and focusing on earlier, more malleable biological features will lead to curative strategies. Cures will provide everyone with asthma the freedom and security to breathe.
Box 1 – The impact and importance of the earliest asthma treatment paradigm shifts, as reported in the Australian Women’s Weekly in 1960*

* Reproduced with permission of Are Media and The Australian Women’s Weekly (CC BY 4.0).
Box 2 – Transitions in asthma treatment concepts, from relief (20th century) to prevention (21st century)*
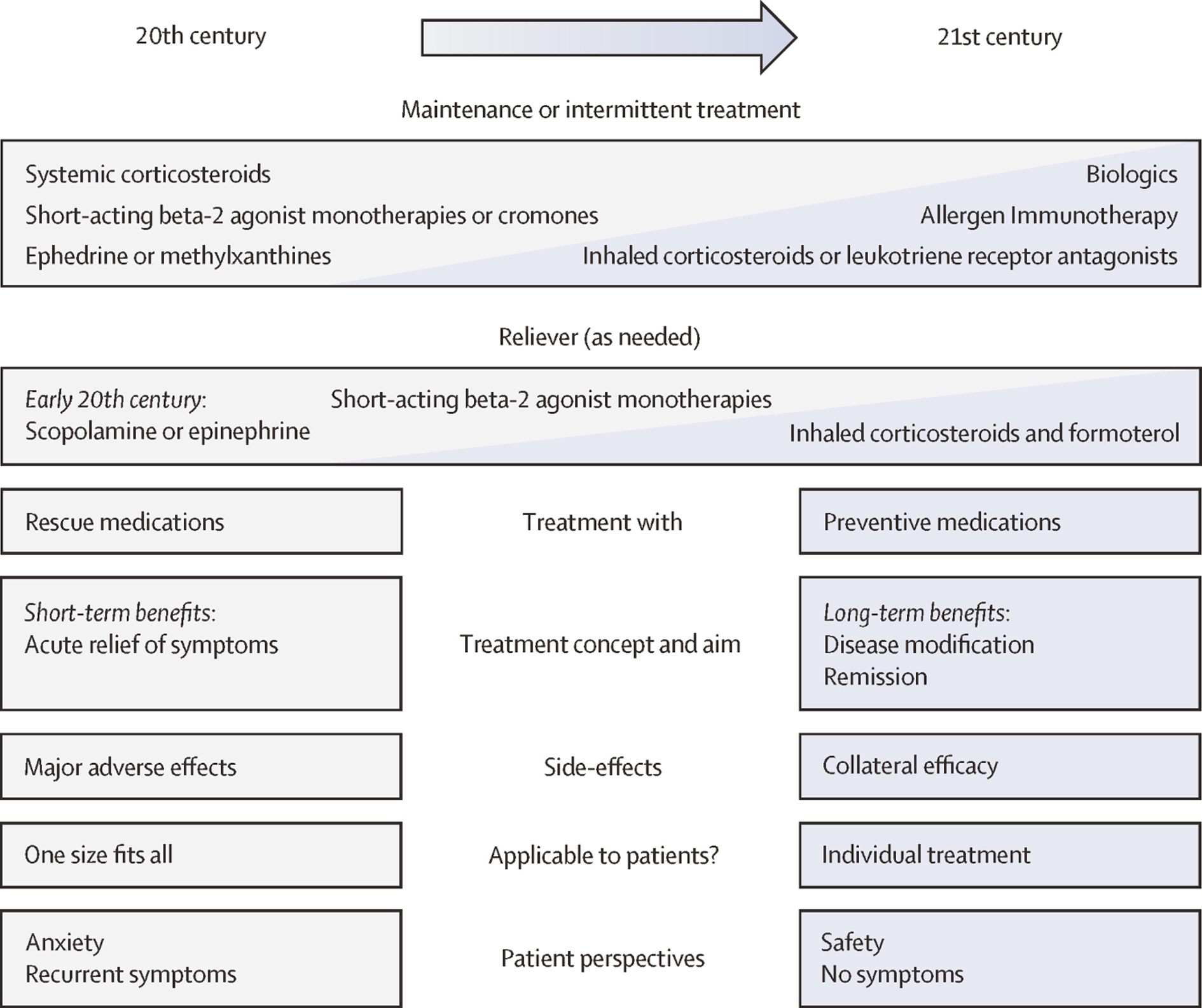
* Source: Lommatzsch and colleagues, 2002.7 Reproduced with permission from Elsevier (CC BY 4.0).
Box 3 – Building on the transitions described in Box 2, our vision is that a pre‐emptive approach before persistent disease is established will lead to asthma cures
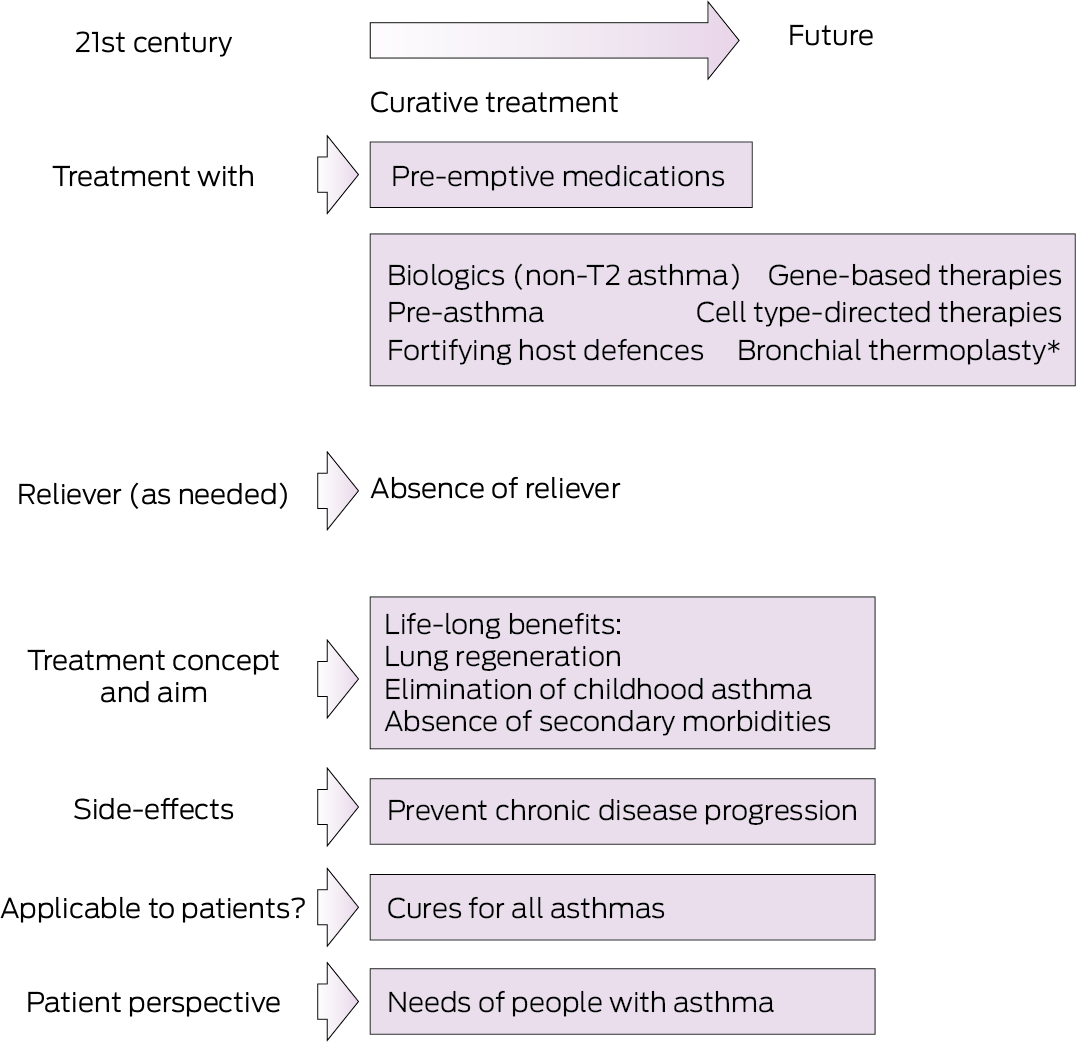
* The manufacturer recently issued a product discontinuation notice for bronchial thermoplasty; its modes of action remain uncertain.15
Provenance: Not commissioned; externally peer reviewed.
- 1. McKie R. Conquering asthma. Australian Women’s Weekly, 6 July 1960, p. 7. Archived: http://nla.gov.au/nla.news‐article56654886 (viewed Oct 2025)
- 2. Mancuso CA, Rincon M, Robbins L, Charlson ME. Patients’ expectations of asthma treatment. J Asthma 2003; 40: 873‐881.
- 3. Walter MJ, Holtzman MJ. A centennial history of research on asthma pathogenesis. Am J Respir Cell Mol Biol 2005; 32: 483‐489.
- 4. Blakey J, Chung LP, McDonald VM, et al. Oral corticosteroids stewardship for asthma in adults and adolescents: a position paper from the Thoracic Society of Australia and New Zealand. Respirology 2021; 26: 1112‐1130.
- 5. Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008; 372: 1107‐1119.
- 6. Centre of Research Excellence Treatable Traits. What are the treatable traits. 2025. https://treatabletraits.org.au/what‐are‐the‐treatable‐traits (viewed Oct 2025).
- 7. Luskin MR, Murakami MA, Manalis SR, Weinstock DM. Targeting minimal residual disease: a path to cure? Nat Rev Cancer 2018; 18: 255‐263.
- 8. Thomas D, McDonald VM, Gibson PG, Kim RY. Defining “cure” for the asthmas. Med J Aust 2025; 223 (10 Suppl): 16‐18.
- 9. Lommatzsch M, Brusselle GG, Canonica GW, et al. Disease‐modifying anti‐asthmatic drugs. Lancet 2022; 399: 1664‐1668.
- 10. Homaira N, Briggs N, Pardy C, et al. Association between respiratory syncytial viral disease and the subsequent risk of the first episode of severe asthma in different subgroups of high‐risk Australian children: a whole‐of‐population‐based cohort study. BMJ Open 2017; 7: e017936.
- 11. Fujiogi M, Dumas O, Hasegawa K, et al. Identifying and predicting severe bronchiolitis profiles at high risk for developing asthma: analysis of three prospective cohorts. eClinicalMedicine 2022; 43: 101257.
- 12. Driscoll AJ, Arshad SH, Bont L, et al. Does respiratory syncytial virus lower respiratory illness in early life cause recurrent wheeze of early childhood and asthma? Critical review of the evidence and guidance for future studies from a World Health Organization‐sponsored meeting. Vaccine 2020; 38: 2435‐2448.
- 13. Quon S, Johanson T, Wadhwa R, et al. Technological advances in the search for a CURE for asthma. Med J Aust 2025; 223 (10 Suppl): 000‐000.
- 14. James AL, Donovan GM, Green FHY, et al. Heterogeneity of airway smooth muscle remodeling in asthma. Am J Respir Crit Care Med 2023; 207: 452‐460.
- 15. Noble PB, Langton D, Foo CT, et al. Beyond bronchial thermoplasty: where to now? eClinicalMedicine 2025; 79: 103017.
- 16. Hagner M, Wurzenberger C, Peper‐Gabriel J, et al. Development of PRS‐400, an inhaled Jagged‐1 Anticalin protein for the treatment of muco‐obstructive lung diseases [abstract: ERS international congress, Barcelona, 4–6 September 2022]. Eur Resp J 2022; 60 (Suppl 66): 931.
- 17. Castro M, Papi A, Porsbjerg C, et al. Effect of dupilumab on exhaled nitric oxide, mucus plugs, and functional respiratory imaging in patients with type 2 asthma (VESTIGE): a randomised, double‐blind, placebo‐controlled, phase 4 trial. Lancet Respir Med 2025; 13: 208‐220.
- 18. Dunican EM, Elicker BM, Gierada DS, et al; National Heart Lung and Blood Institute (NHLBI) Severe Asthma Research Program (SARP). Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest 2018; 128: 997‐1009.
- 19. Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 2010; 363: 2233‐2247.





None.
Dennis Thomas has received grants from GlaxoSmithKline, unrelated to this article. Gary P Anderson has received personal fees from AstraZeneca, GlaxoSmithKline, ENA Respiratory, RAGE Biotech, DevPro, and Pieris Pharmaceuticals, and research grants from RAGE Biotech, unrelated to this article. Gary P Anderson is a co‐founder of ARI‐Tx, which is developing an inhaled protein therapy that targets JAG‐1 in muco‐obstructive lung diseases, including asthma.
Author contribution:
All authors contributed to the conceptualisation, preparation of the initial draft, and review and editing of the final version of the manuscript.