Zoonotic infections account for a small proportion of hospital presentations, even when focusing solely on the eastern states of Australia.1,2 In rare instances, however, these infections can progress to severe, life‐threatening complications. The cases presented here underscore the importance of including zoonotic infections in the differential diagnosis of patients with unexplained sepsis and maintaining a high index of suspicion for potential complications. Serological testing for zoonotic pathogens can be slow to return results, particularly when sent from regional or remote areas. Given this delay, we recommend continuing empirical treatment when clinical suspicion for a zoonotic infection is high, pending confirmation through diagnostic testing.
In accordance with the Sex and Gender Equity in Research (SAGER) guidelines,3 we have reported the sex of our patients, defined here based on biological characteristics. We have followed the United Nations’ definition of sex as referring to the physical and biological traits that distinguish males and females. Both cases described in this report involve male patients.
Case One
A 45‐year‐old man presented to a regional New South Wales emergency department with five days of malaise, lethargy and dyspnoea. His history included hypertension, haemochromatosis and gastro‐oesophageal reflux disease. He reported abdominal discomfort, vomiting and a tick bite three weeks prior, associated with erythema and cervical lymphadenopathy but no overt eschar nor rash.
On examination, he was febrile (39.9°C), tachycardic, hypotensive and hypoxic. His abdomen was soft but diffusely tender. Initial investigations demonstrated metabolic acidosis, acute kidney injury, deranged liver function tests, thrombocytopaenia, lymphopenia, coagulopathy and raised inflammatory markers (Box 1). A lumbar puncture was deferred due to thrombocytopaenia. A computed tomography (CT) scan of the abdomen and pelvis was unremarkable.
He was admitted to the intensive care unit with sepsis, requiring vasopressor support. Empirical treatment included intravenous ceftriaxone (2 g every 12 hours) and oral doxycycline (100 mg every 12 hours) to cover potential zoonoses. A septic screen, including five sets of blood cultures, was negative. A lumbar puncture on day 10 demonstrated cerebrospinal fluid counts of 15 × 106/L white cells (reference interval [RI], 4–11 × 106/L; 100% mononuclear), 3 × 106/L red cells (RI, 0–5 × 106/L) with a glucose of 4.1 mmol/L (RI, 2.5–4.4 mmol/L) and protein of 1.67 g/L (RI, 0.2–0.59 g/L). There was no growth on culture, and no pathogens were detected on an extended molecular testing panel.
He completed seven days of broad‐spectrum antibiotics, and doxycycline was continued pending serology. A serial CT scan of the chest, abdomen and pelvis was performed to further investigate the cause of sepsis and demonstrated an incidental grade four splenic injury with peri‐splenic and subcapsular collections (Box 2). With a stable haemoglobin level, he was managed conservatively.
He completed 14 days of doxycycline and improved clinically. Serology confirmed spotted fever group Rickettsia with rising titres (from 512 to 32 768). He returned for vascular operative management of his gangrenous lower limbs and required a left forefoot amputation five weeks after initial presentation.
Case Two
A 59‐year‐old man presented with six days of fevers, chills, rigors, headache, arthralgia, anorexia and nausea. He lived in regional NSW with no animal exposures nor recent insect bites. He worked as a plasterer, smoked about 20 cigarettes per day and consumed four standard alcoholic drinks every night. He had not previously been vaccinated against Q fever. He was febrile (39.4°C) and mildly photophobic. His abdomen was soft but tender in the right upper quadrant and epigastrium. He had palpable hepatosplenomegaly without lymphadenopathy nor a rash.
Investigations revealed hyponatraemia, acute kidney injury, deranged liver function tests, lymphopenia, thrombocytopaenia and a raised C‐reactive protein (Box 3). A lumbar puncture was contraindicated due to thrombocytopaenia. A CT scan of the abdomen and pelvis demonstrated hepatosplenomegaly and pulmonary ground glass opacities.
Five sets of blood cultures had no growth. A sputum culture grew scant oropharyngeal flora, and urine antigen for Legionella pneumophila type 1 was negative. Empirical therapy, with intravenous benzylpenicillin (1.2 g every 6 hours) and oral doxycycline (100 mg every 12 hours), was started for moderate community‐acquired pneumonia.
Over the next four days, the patient continued to have daily fevers above 39°C and experience abdominal pain. He underwent a repeat CT scan of the abdomen and pelvis, which demonstrated splenomegaly with a grade three to four splenic injury and surrounding peri‐splenic collection.
The spontaneous splenic injury was managed conservatively. The haemoglobin level dropped to a nadir of 68 g/L (RI, 130–170 g/L) and required two transfusions of packed red blood cells. Q fever serology returned a result of phase 2 IgM titre of 3072 and phase 2 IgG titre of 128. Convalescent serology demonstrated a phase 2 IgM titre of 49 152 and phase 2 IgG titre of 8192, confirming the diagnosis of acute Q fever.
Discussion
These two regional NSW cases highlight rare but serious complications of zoonotic infections: spontaneous splenic injury due to spotted fever group Rickettsia and Q fever.
Rickettsia are obligate intracellular, gram‐negative bacteria. In Australia, spotted fever group rickettsial infection may be caused by Rickettsia australis, Rickettsia honei, or Rickettsia honei subsp. marmionii. These organisms share clinical features and have geographical overlap.2 Although typically presenting with fever, headache, rash and myalgia, severe complications of rickettsial infection are becoming increasingly recognised.4,5 Severe but uncommon complications include acute kidney injury, multiorgan failure, purpura fulminans, coagulopathy, and respiratory failure.5 In a case series from Queensland, of the 22 patients who required intensive care unit admission, splenomegaly was only reported in one patient (5%) and hepatomegaly in two patients (9%).6 Spontaneous splenic injury is rarely associated with rickettsial infection.7,8
Coxiella burnetii, the causative agent of Q fever, is also an intracellular, gram‐negative bacterium, primarily transmitted via inhalation. It causes a non‐specific febrile illness, often with myalgia, headache, fatigue and nausea.9,10 Complications include hepatitis, pneumonia and endocarditis.9,10 Splenic and hepatic abscesses and subsequent splenic injury have been reported as rare manifestations of Q fever.10,11,12,13,14,15,16
The pathophysiology of atraumatic splenic injury has not been elucidated.17,18 Proposed mechanisms include increased splenic tension due to congestion, vascular anomaly or enzymic digestion of the capsule.17 Many patients with atraumatic splenic injury have splenomegaly; however, this is often unknown as the patient may not have had prior abdominal imaging.18
Definitive diagnosis is often delayed while awaiting serology; therefore, polymerase chain reaction (PCR) testing for both pathogens is an important part of the diagnostic workup. In addition, a negative PCR test result in early infection does not definitively rule out these pathogens, so serology is always recommended. Absence of rash or eschar (present in only 7% of patients in one case series) may reduce clinical suspicion of rickettsial infection.19 Both rickettsial infection and Q fever require a high index of suspicion, especially in endemic areas, and respond well to doxycycline.20 Early doxycycline use is associated with reduced fever duration in both of these zoonoses.21
Lessons from practice
- Zoonotic infections, including Q fever and rickettsial infections, should be considered in cases of unexplained sepsis in Australia.
- Splenic injury, though rare, is a potentially lethal complication of both diseases and should be suspected in patients with abdominal pain and splenomegaly.
- Empirical therapy should be initiated and continued when a zoonotic infection is suspected, pending diagnostic testing.
- Polymerase chain reaction testing provides earlier diagnosis than serology and should be sent where clinical suspicion is high, given that serology alone may miss early infection.
Box 1 – Laboratory values on presentation
|
Test |
Result (reference interval) |
Interpretation |
|||||||||||||
|
|
|||||||||||||||
|
Lactate, mmol/L |
6.1 (< 1) |
High |
|||||||||||||
|
Creatinine, μmol/L |
209 (60–110) |
High |
|||||||||||||
|
eGFR, mL/min |
32 (> 60) |
Low |
|||||||||||||
|
Bilirubin, μmol/L |
106 (≤ 20) |
High |
|||||||||||||
|
GGT, unit/L |
540 (5–50) |
High |
|||||||||||||
|
ALP, unit/L |
224 (30–110) |
High |
|||||||||||||
|
ALT, unit/L |
99 (10–50) |
High |
|||||||||||||
|
AST, unit/L |
153 (10–35) |
High |
|||||||||||||
|
WCC, × 109/L |
7.0 (4–11) |
Normal |
|||||||||||||
|
Lymphocytes, × 109/L |
0.2 (1–4) |
Low |
|||||||||||||
|
Platelet count, × 109/L |
28 (150–400) |
Low |
|||||||||||||
|
CRP, mg/L |
257 (≤ 5) |
High |
|||||||||||||
|
Procalcitonin, μg/L |
10.98 (< 0.5) |
High |
|||||||||||||
|
PT, seconds |
19 (12–15) |
High |
|||||||||||||
|
APTT, seconds |
38 (25–37) |
High |
|||||||||||||
|
Fibrinogen, g/L |
0.4 (2–4.6) |
Low |
|||||||||||||
|
|
|||||||||||||||
|
ALP = alkaline phosphatase; ALT = alanine aminotransferase; APTT = activated partial thromboplastin time; AST = aspartate aminotransferase; CRP = C‐reactive protein; eGFR = estimated glomerular filtration rate; GGT = γ‐glutamyl transferase; PT = prothrombin time; WCC = white cell count. |
|||||||||||||||
Box 2 – Grade four splenic injury with peri‐splenic and subcapsular collections
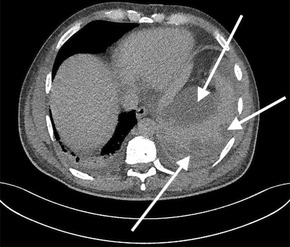
*The arrows indicate the splenic injury.
Box 3 – Laboratory values on presentation
|
Test |
Result (reference interval) |
Interpretation |
|||||||||||||
|
|
|||||||||||||||
|
Sodium, mmol/L |
131 (135–145) |
Low |
|||||||||||||
|
Creatinine, μmol/L |
151 (60–110) |
High |
|||||||||||||
|
eGFR, mL/min |
43 (> 60) |
Low |
|||||||||||||
|
Bilirubin, μmol/L |
24 (≤ 20) |
High |
|||||||||||||
|
GGT, unit/L |
68 (5–50) |
High |
|||||||||||||
|
ALP, unit/L |
196 (30–110) |
High |
|||||||||||||
|
ALT, unit/L |
78 (10–50) |
High |
|||||||||||||
|
Lymphocytes, × 109/L |
0.4 (1–4) |
Low |
|||||||||||||
|
Platelet count, × 109/L |
29 (150–400) |
Low |
|||||||||||||
|
CRP, mg/L |
166 (≤ 5) |
High |
|||||||||||||
|
|
|||||||||||||||
|
ALP = alkaline phosphatase; ALT = alanine aminotransferase; CRP = C‐reactive protein; eGFR = estimated glomerular filtration rate; GGT = γ‐glutamyl transferase. |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- 1. Hamad G, Ranmuthugala G. Q fever awareness in Australia: a scoping review. Aust N Z J Public Health 2023; 47: 100099.
- 2. Stewart A, Armstrong M, Graves S, et al. Rickettsia australis and Queensland tick typhus: a rickettsial spotted fever group infection in Australia. Am J Trop Med Hyg 2017; 97: 24‐29.
- 3. European Association of Science Editors Gender Policy Committee. Sex and Gender Equity in Research guidelines. EASE Publications. https://ease.org.uk/communities/gender‐policy‐committee/the‐sager‐guidelines/ (viewed Sept 2025).
- 4. Dehhaghi M, Kazemi Shariat Panahi H, Holmes EC, et al. Human tick‐borne diseases in Australia. Front Cell Infect Microbiol 2019; 9: 3.
- 5. McBride W, Hanson J, Miller R, et al. Severe spotted fever group rickettsiosis, Australia. Emerg Infect Dis 2007; 13: 1742‐1744.
- 6. Bagshaw R, Stewart A, Smith S, et al. The characteristics and clinical course of patients with scrub typhus and Queensland tick typhus infection requiring intensive care unit admission: a 23‐year case series from Queensland, Tropical Australia. Am J Trop Med Hyg 2020; 103: 2472‐2477.
- 7. Schmulewitz L, Moumile K, Patey‐Mariaud de Serre N, et al. Splenic rupture and malignant Mediterranean spotted fever. Emerg Infect Dis 2008; 14: 995‐997.
- 8. Thipmontree W, Suwattanabunpot K, Supputtamonkol Y. Spontaneous splenic rupture caused by scrub typhus. Am J Trop Med Hyg 2016; 95: 1284‐1286.
- 9. Eldin C, Mélenotte C, Mediannikov O. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev 2016; 16: 115‐190.
- 10. Gomes MM, Chaves A, Gouveia A, Santos L. Two rare manifestations of Q fever: splenic and hepatic abscesses and cerebral venous thrombosis, with literature review ma non troppo. BMJ Case Rep 2014; bcr2013202843.
- 11. Wade A, Walker T, Athan E, et al. Spontaneous splenic rupture: a rare complication of Q fever in Australia. Med J Aust 2006; 184: 364. https://www.mja.com.au/journal/2006/184/7/spontaneous‐splenic‐rupture‐rare‐complication‐q‐fever‐australia
- 12. Baumbach A, Brehm B, Sauer W, et al. Spontaneous splenic rupture complicating acute Q fever. Am J Gastroenterol. 1992; 87: 1651‐1653.
- 13. Henderson SA, Templeton JL, Wilkinson AJ. Spontaneous splenic rupture: a unique presentation of Q fever. Ulster Med J 1988; 57: 218‐219.
- 14. Kazemy AH. Spontaneous rupture of spleen due to Q fever. South Med J 2000; 93: 609‐610.
- 15. Serrano‐Herranz R, Ibáñez Pérez R, Francos Von Hunefeld M, Gil Yonte P. [Spontaneous spleen rupture and Q fever] [Spanish]. Rev Clin Esp 2002; 202: 123.
- 16. Millán Rodríguez AB, Domínguez Castellano Á, Ramírez de Arellano E, Muniaín Ezcurra MÁ. [Q fever and spontaneous splenic rupture] [Spanish]. Med Clin (Barc) 2005; 124: 796‐797.
- 17. Alyaseen EM, Hantol NA, Alyousef JA, et al. Spontaneous splenic rupture in a young patient: a comprehensive case report and literature review. Cureus 2024; 16: e60105.
- 18. Renzulli P, Hostettler A, Schoepfer A, et al. Systematic review of atraumatic splenic rupture. Br J Surg 2009; 96: 1114‐1121.
- 19. Graves S, Islam A. Epidemiological and clinical features of rickettsial infection among Australian individuals from 2015 to 2018. Zoonoses. 2024; 4: 31.
- 20. Lee N, Ip M, Wong B, et al. Risk factors associated with life‐threatening rickettsial infections. Am J Trop Med Hyg 2008; 786: 973‐978.
- 21. Clay A, Hartley M, Armstrong S, et al. Evaluation of the efficacy of doxycycline, ciprofloxacin, levofloxacin, and co‐trimoxazole using in vitro and in vivo models of Q fever. Antimicrob Agents Chemother 2021; 65: e0067321.





Patient consent:
The patients provided written consent for publication.
No relevant disclosures.
Author contributions:
Ashleigh Drury: Conceptualization, investigation, writing – original draft, writing – review and editing. Philippa Harrison: Conceptualization, investigation, writing – original draft. Aiveen Bannan: Supervision.