The known: COVID‐19 vaccines protect against severe disease, but less is known about the effectiveness of monovalent XBB.1.5 vaccine boosters for protecting against COVID‐19 death in Australia.
The new: During August 2023 – February 2024, XBB.1.5 vaccine boosters during the preceding three months reduced the likelihood of COVID‐19 death among Australians aged 65 years or older by 75%, compared with not having received a booster for more than twelve months.
The implications: COVID‐19 boosters continue to provide significant protection against death from SARS‐CoV‐2 infections. Regular vaccine boosting saves lives, particularly those of older Australians, who are at greatest risk of death from COVID‐19.
The pathogen of coronavirus disease 2019 (COVID‐19), the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), continues to evolve, and immune evasion is associated with considerable morbidity and mortality, despite widespread vaccination against the virus. In response, the World Health Organization Technical Advisory Group on COVID‐19 Vaccine Composition recommended in May 2023 a monovalent XBB.1 lineage variant as the antigen for vaccine formulations.1 In November 2023, the monovalent XBB.1.5 mRNA COVID‐19 vaccine was registered in Australia for primary or booster vaccinations of all people aged 12 years or older.2
The XBB.1.5 formulation is effective for preventing infection and hospitalisations caused by SARS‐CoV‐2 variants that circulated during 2023.3,4 Some, but not all, studies have found it is more effective for preventing disease caused by XBB lineage viruses than infections with JN.1 lineage variants (descended from BA.2.86).3,5,6,7,8 Data regarding the effectiveness of the XBB.1.5 vaccine against death from COVID‐19 are more limited.6,9 The waning of COVID‐19 booster effectiveness also continues to be reported,6,7 but the effects of changes in viral subvariants over time and declines in individual antibody levels and immune responsiveness are difficult to disentangle because these factors are temporally related.
The Australian COVID‐19 vaccination program has focused on preventing serious illness and death. Six‐monthly COVID‐19 boosters are recommended for people aged 75 years or older, and annual vaccination for people aged 65–74 years; 6‐monthly boosters should also be considered by people aged 65–74 years and all adults with severe immunocompromise.10 The use of XBB 1.5 vaccines produced by Pfizer and Moderna was recommended in Australia on 20 November 2023.2
As the evidence regarding XBB.1.5 vaccine effectiveness for preventing COVID‐19‐related death is limited, we examined the effectiveness of COVID‐19 booster vaccine doses, time since most recent booster, and booster vaccine type in adults aged 65 years or older in Australia during August 2023 – February 2024, a period initially dominated by XBB‐related SARS‐CoV‐2 Omicron subvariants, including EG.5, and then by the JN.1 subvariant.5
Methods
We have previously described the data sources and methods we use to assess COVID‐19 vaccine effectiveness for preventing COVID‐19‐related deaths.11 Briefly, a whole of population cohort was established for our retrospective observational cohort study by linking Australian 2021 census data to the Australian Immunisation Register (AIR) and national death registrations using the Person Level Integrated Data Asset (PLIDA) managed by the Australian Bureau of Statistics.12 The census data include information on people living in more than 96% of Australian dwellings. The AIR records all vaccinations undertaken in Australia; the reporting of COVID‐19 vaccinations is mandatory. National death registrations include records for all deaths registered in Australia, including the contributing causes of death, coded according to the International Classifications of Diseases, tenth revision (ICD‐10). Other linked data, including the Medicare Benefits Schedule and Pharmaceutical Benefits Scheme databases, provided information about health service and medication use. For the analysis reported in this article, we included all people aged 65 years or older included in 2021 census data who had not emigrated or died before 1 August 2023. De‐identified data were supplied to the authors; following analyses, all results are perturbed according to Australian Bureau of Statistics methods for preventing the disclosure of small numbers and potential identification of individual persons.
The study outcome was COVID‐19 death, defined by a death registration in which the underlying cause of death was recorded with ICD‐10 code U07.1 or U07.2. We classified people by COVID‐19 vaccination history. At January 2024, most people aged 65 years or older in Australia had received at least one vaccine dose during the preceding twelve months;13 people without significant immunocompromising conditions could have received as many as seven COVID‐19 vaccine doses (two for the primary course, and five boosters). We therefore classified and compared vaccination status by time since most recent booster dose (more than 365 days [reference group], 181–365 days, 91–180 days, 8–90 days). As the XBB.1.5 vaccine became available in November 2023, we also classified people who had received boosters during the past 8–90 days according to whether they received the XBB.1.5 monovalent vaccine or another COVID‐19 booster type.
Individuals were followed from 1 August 2023 to 29 February 2024 for COVID‐19‐specific death, censored at the date of death, date of an eighth COVID‐19 vaccine dose, or 29 February 2024, whichever was earliest. The relationship between time since most recent booster dose and mortality was assessed in Cox proportional hazards models adjusted for age, gender (derived from various linked data sources, including 2021 census data; provided to the research team in the demographic characteristics file), residential state or territory, weekly household income bracket, number of medical conditions during the preceding six months (Rx‐Risk Comorbidity Index, based on pharmaceuticals prescribing),14 number of general practice visits during the preceding twelve months (as a measure of health service use), and influenza vaccination during 2022; COVID‐19 vaccination status was included as a time‐varying covariate. We estimated adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs), and relative vaccine effectiveness was calculated as (1 – aHR)*100%. Crude COVID‐19‐related mortality rates (per 100 000 person‐years) were calculated using unadjusted quasi‐Poisson regression, and absolute risks of death by time since most recent COVID‐19 vaccine booster and age group (65 years or older, 75 years or older) were calculated by multiplying the crude rates for the reference group by the adjusted hazard ratios. In sensitivity analyses, follow‐up was restricted to the period 1 December 2023 – 29 February 2024, when JN.1 SARS‐CoV‐2 subvariants dominated in Australia.5
Ethics approval
As the reported analyses were conducted as part of the monitoring of the Australian government COVID‐19 vaccination program, the Sydney Children’s Hospital Human Research Ethics Committee exempted the surveillance study from formal ethics review.
Results
We included 4.12 million people aged 65 years or older on 1 August 2023 in our analyses; 1.89 million (45.8%) were aged 75 years or older, 2.21 million (53.7%) were women, 2.47 million (59.8%) had household incomes of less than $1000 per week, 1.02 million (24.8%) had six or more medical conditions, 1.28 million (31.0%) had made thirteen or more visits to general practitioners in the preceding 365 days, and 3.19 million (77.4%) had received influenza vaccines during 2022 (Supporting Information, table 1).
On 1 August 2023, 459 049 people aged 65 years or older (11%) were unvaccinated or had received one or two COVID‐19 vaccine doses; on 29 February 2024, the number was 455 833 (11%). The number of people who had received six or seven vaccine doses increased from 37 994 (0.9%) on 1 August to 798 635 (18.8%) by 29 February 2024 (Supporting Information, figure 1).
By 29 February 2024, 3.66 million people aged 65 years or older had received at least three COVID‐19 vaccine doses, 2.99 million at least four doses, 2.09 million at least five doses, 798 635 at least six doses, and 23 996 seven doses. The monovalent XBB.1.5 mRNA formulation was used for 464 947 dose 6 or 7 boosters (56.5%); the bivalent ancestral and BA4/5 mRNA vaccine was used for 1 856 773 dose 5 boosters (88.6%); the original ancestral monovalent mRNA vaccine was used for 6 329 786 dose 3 and 4 boosters (95.1%) (Supporting Information, figure 2).
During 2.37 million person‐years of follow‐up, 1620 people died of COVID‐19. The crude COVID‐19 mortality rate was lower among people who had received an XBB.1.5 COVID‐19 booster dose during the preceding 90 days (21 [95% CI, 9–52] per 100 000 person‐years) than among those whose most recent booster had been more than 365 days ago (72 [95% CI, 61–84] per 100 000 person‐years). The relative vaccine effectiveness for XBB.1.5 boosters during the preceding 90 days (v any booster > 365 days) was 74.7% (95% CI, 59.9–84.1%); for other booster types during the preceding 90 days, the relative vaccine effectiveness was 51.6% (95% CI, 39.3–61.4%) (Box 1).
Among people who received booster doses during the preceding 90 days (during follow‐up when both vaccines were available), the mean median time since receiving the dose was 28.8 (standard deviation, 12.5) days for people who received XBB.1.5 boosters and 59.0 (standard deviation, 17.0) days for those who received other booster vaccine types (Supporting Information, figure 3). Relative vaccine effectiveness declined with time since the booster dose: for any booster during the preceding 91–180 days (v any booster > 365 days) it was 31.2% (95% CI, 18.9–41.6%), and for any booster during the preceding 181–365 days it was 13.1% (95% CI, 1.8–23.2%). The overall relative vaccine effectiveness for receiving any booster during the preceding 8–180 days (the recommended booster interval for people aged 75 years or older in Australia) was 44.1% (95% CI, 35.6–51.4%).
For people aged 65 years or older who had not received COVID‐19 boosters during the preceding 365 days, the adjusted absolute risk of death from COVID‐19 was 0.72 per 1000 person‐years (aged 75 years or older: 1.5 per 1000 person‐years); for those who had received XBB.1.5 booster during the preceding 90 days, the adjusted absolute risk was 0.18 per 1000 person‐years (aged 75 years or older: 0.36 per 1000 person‐years) (Box 2).
The relative vaccine effectiveness for people aged 75 years or older were similar to that for people aged 65 years or older, but the crude COVID‐19 mortality rates were substantially higher: 153 (95% CI, 130–180) per 100 000 person‐years for those who had not received boosters during the preceding 365 days and 40 (95% CI, 16–104) per 100 000 person‐years for people who had received XBB.1.5 boosters during the preceding 90 days (Box 3).
The estimated relative vaccine effectiveness values were similar to those in the main analysis in sensitivity analyses restricted to follow‐up during 1 December 2023 – 29 February 2024 (Box 4). Post hoc analyses that included death from other causes as a competing risk yielded similar relative vaccine effectiveness values to the main analysis (data not shown).
Discussion
Monitoring the effectiveness of new COVID‐19 vaccines is particularly important given the continuing evolution of SARS‐CoV‐2. We found that the likelihood of death from COVID‐19 among adults aged 65 years or older was 74.7% lower for people who had received XBB.1.5 vaccine boosters during the past three months than for those who last received any booster more than 365 days ago; with other vaccine booster types, the reduction was 51.6%. The estimated relative vaccine effectiveness values were similar for people aged 75 years or older (XBB.1.5 vaccine boosters, 76.7%; other boosters, 50.0%), but the absolute benefits in terms of reduced mortality rates were substantially greater. We also found that relative vaccine effectiveness was similar during December 2023 – February 2024, when the dominant circulating sub‐variant was JN.1 (of the BA.2.86 lineage).5
Recent studies in the United States, United Kingdom, and Europe have consistently found that XBB.1.5 variant vaccines are effective in the short term for protecting people against SARS‐CoV‐2 infection and hospitalisation with COVID‐19.3,4,6,7,8,9,15 Fewer studies have examined COVID‐19 mortality; a large Nordic analysis suggested that the vaccine was probably more effective in protecting against death than against hospitalisation.8 Findings regarding the waning of XBB.1.5 vaccine effectiveness for preventing hospitalisation with COVID‐19 and against specific SARS‐CoV‐2 subvariants are less consistent. One study found that effectiveness waned considerably over three months,7 another did not.8 Further, the effectiveness of the XBB.1.5 vaccine for protecting against infection and hospitalisation is reported to be lower against the JN.1 sub‐lineage than XBB SARS‐CoV‐2 lineages,3,6 but not by all studies.8 However, the characteristics of vaccine effectiveness, such as waning, are difficult to study because changes in circulating viral variants will coincide with increasing time since a variant‐specific vaccine was introduced into a vaccination program and population.
While direct comparisons are difficult because of differences in the comparator groups, our estimate of the relative effectiveness of the XBB.1.5 vaccine with respect to COVID‐19 mortality was similar to that reported by other studies. Effectiveness with respect to hospitalisation, for similar times since the most recent booster dose, has been reported to be 55–67%,6,8,9,15 and 67–78% with respect to death from COVID‐19.8,9 We found that the relative effectiveness of the XBB.1.5 vaccine for protecting against death from COVID‐19 was similar during December 2023 – February 2024, when the JN.1 subvariant was dominant in Australia, but the XBB.1.5 vaccine only became available in Australia during this period.
Our estimates of the relative effectiveness of boosters during the preceding 8–90 days indicated that other booster types (mostly the bivalent mRNA vaccines) were less effective than the XBB.1.5 vaccine. A difference in effectiveness was also reported by another study,7 but it was not statistically significant. Among people who received their most recent booster dose during the preceding 8–90 days, the median time since that dose was 32 days shorter for those who received the XBB.1.5 vaccine than for people who received other booster types. As we and other authors7 have reported waning of mRNA COVID‐19 vaccine effectiveness, the difference between vaccine types might be explained by waning effectiveness with time rather than differences in the effectiveness of vaccine formulations.
Limitations
Our analysis was based on large national datasets, including census and migration data and the Australian Immunisation Register; reporting of COVID‐19 vaccinations to the Registry has been mandatory since the COVID‐19 vaccination program commenced in March 2021. Death registrations based on death certification avoid some, but not all biases associated with COVID‐19‐related hospitalisations as an outcome measure because ascertainment has changed over time with changes in testing, reporting, and disease severity. The limitations of observational studies of COVID‐19 vaccine effectiveness include unmeasured and residual confounding by factors such as healthy vaccinee effects, the likelihood that people with other medical conditions will more frequently receive booster vaccine doses, health service access, and other socio‐demographic characteristics. We minimised these effects by adjusting our analyses for potential confounders for which data were available in the datasets, and by estimating relative vaccine effectiveness.
Conclusions
We found that the XBB.1.5 variant vaccine was highly effective during August 2023 – February 2024 as a booster for protecting people aged 65 years or older from death from COVID‐19. The effectiveness of COVID‐19 boosters waned over time; the effectiveness of boosters was almost as low after six months as after twelve months. Based on our findings and those in other reports, providing vaccine boosters at 6‐monthly intervals to people at greater risk of serious disease, as recommended in Australia, will significantly reduce COVID‐19 mortality, particularly among people aged 75 years or older. Following updated recommendations regarding future COVID‐19 vaccine formulations,16 we have undertaken more timely assessments of their effectiveness for preventing severe outcomes such as death. Continued surveillance of vaccine effectiveness, including for preventing hospitalisation and intensive care admission, is important for ensuring our vaccination programs are based on the best evidence.
Box 1 – COVID‐19 mortality rate and relative vaccine effectiveness* of COVID‐19 vaccine boosters for protecting people in Australia aged 65 years or older against COVID‐19 death, 1 August 2023 – 29 February 2024, by time since most recent booster dose
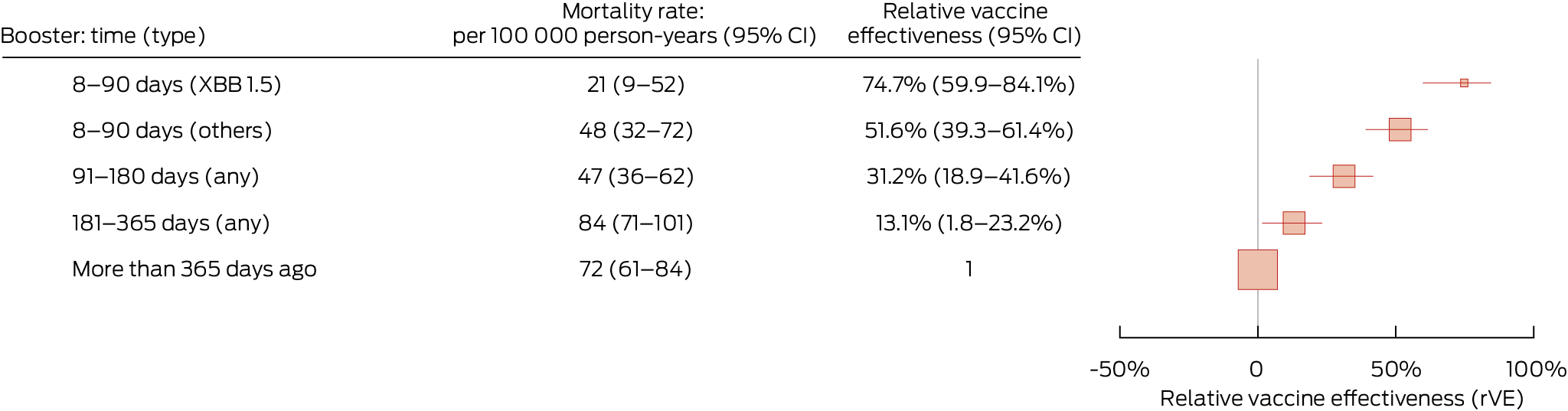
CI = confidence interval; COVID‐19 = coronavirus disease 2019. * Adjusted for age, gender, residential state or territory, weekly household income bracket, number of medical conditions during the preceding six months, number of general practice visits during the preceding twelve months, and influenza vaccination during 2022. There were 1620 deaths during total follow‐up of 2 372 157 person‐years. Boosters include COVID‐19 vaccine doses 3 to 7.
Box 2 – Adjusted COVID‐19 mortality* among people in Australia aged 65 years or older, 1 August 2023 – 29 February 2024, by age and time since most recent COVID‐19 vaccine booster
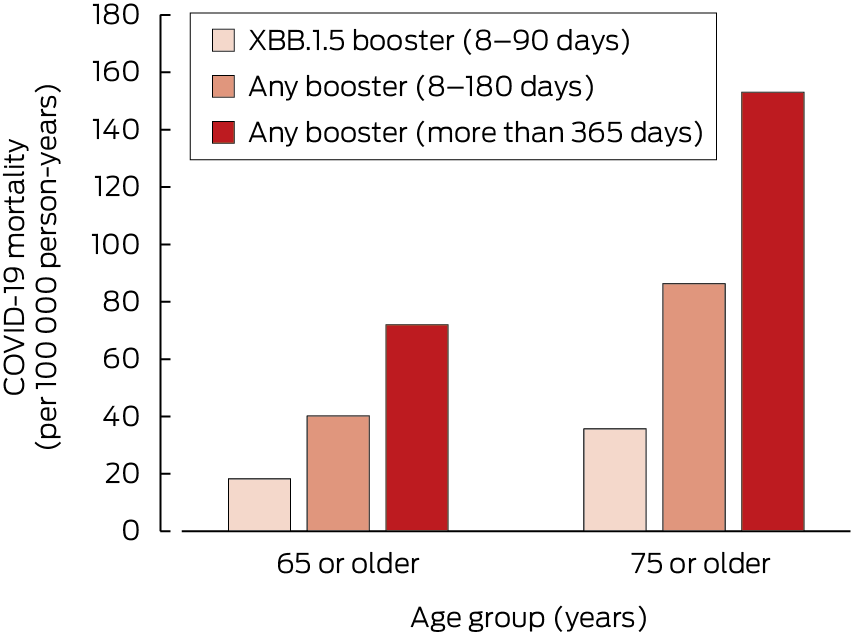
COVID‐19 = coronavirus disease 2019. * Adjusted for age, gender, residential state or territory, weekly household income bracket, number of medical conditions during the preceding six months, number of general practice visits during the preceding twelve months, and influenza vaccination during 2022.
Box 3 – COVID‐19 mortality rate and relative vaccine effectiveness* of COVID‐19 vaccine boosters for protecting people in Australia aged 75 years or older against COVID‐19 death, 1 August 2023 – 29 February 2024, by time since most recent booster dose
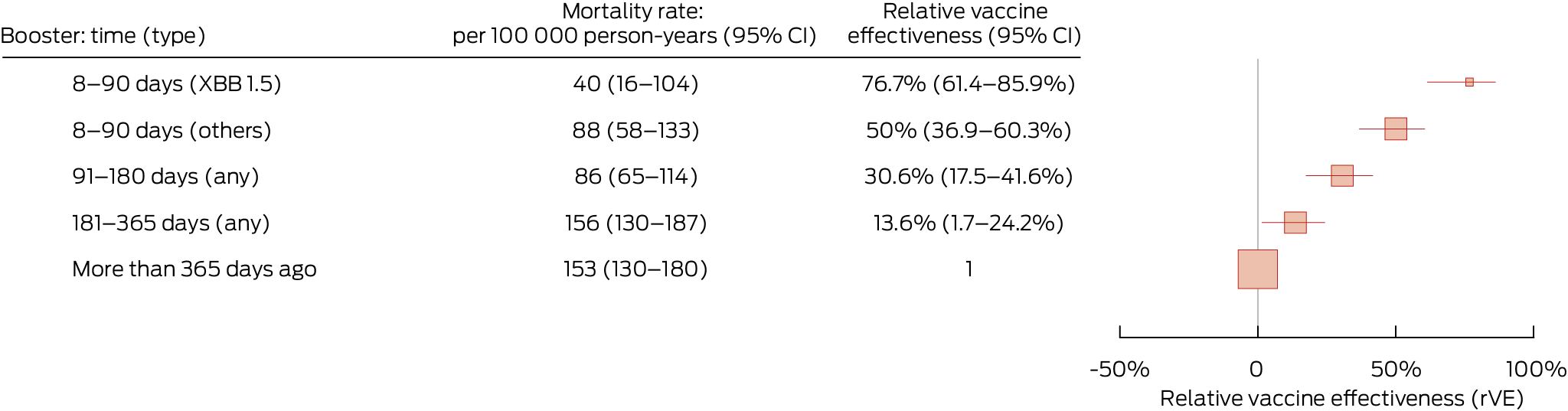
CI = confidence interval; COVID‐19 = coronavirus disease 2019. * Adjusted for age, gender, residential state or territory, weekly household income bracket, number of medical conditions during the preceding six months, number of general practice visits during the preceding twelve months, and influenza vaccination during 2022. There were 1435 deaths during total follow‐up of 1 078 436 person‐years. Boosters include COVID‐19 vaccine doses 3 to 7.
Box 4 – COVID‐19 mortality rate and relative vaccine effectiveness* of COVID‐19 vaccine boosters for protecting people in Australia aged 65 years or older against COVID‐19 death, 1 December 2023 – 29 February 2024, by time since most recent booster dose
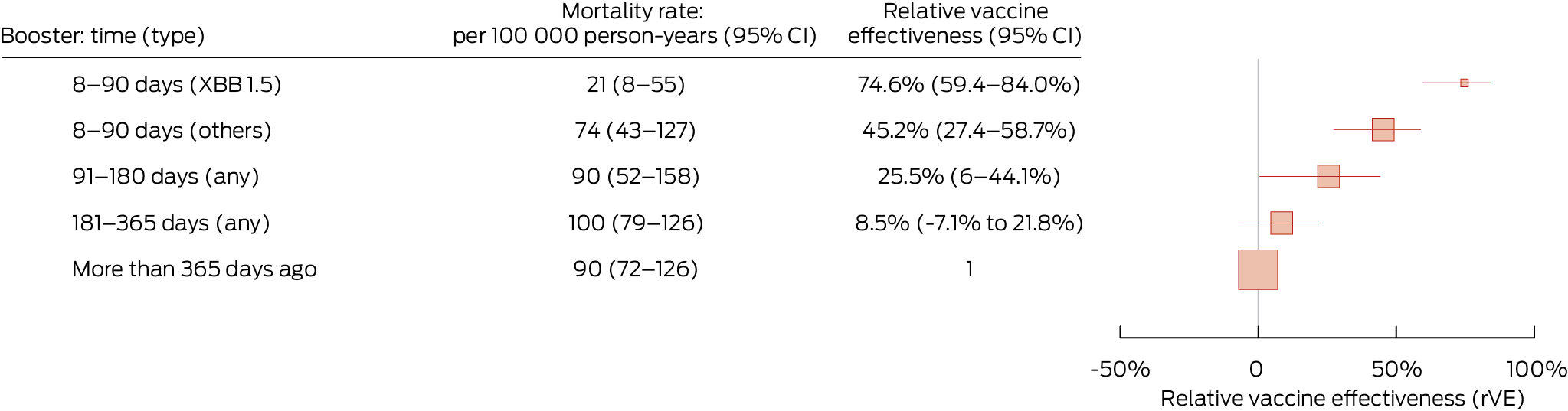
CI = confidence interval; COVID‐19 = coronavirus disease 2019. * Adjusted for age, gender, residential state or territory, weekly household income bracket, number of medical conditions during the preceding six months, number of general practice visits during the preceding twelve months, and influenza vaccination during 2022. There were 870 deaths during total follow‐up of 1 001 822 person‐years. Boosters include COVID‐19 vaccine doses 3 to 7.
Received 7 August 2024, accepted 26 March 2025
- Bette Liu1,2
- Anish Scaria1
- Sandrine Stepien1
- Kristine Macartney1,3
- 1 National Centre for Immunisation Research and Surveillance (NCIRS), Children’s Hospital at Westmead, Sydney, NSW
- 2 University of New South Wales, Sydney, NSW
- 3 The University of Sydney, Sydney, NSW
Correspondence: bette.liu@unsw.edu.au
Open access:
Open access publishing facilitated by the University of New South Wales, as part of the Wiley – University of New South Wales agreement via the Council of Australian University Librarians.
Data Sharing:
This study did not generate any original data.
The study was funded by the Health Economics Research Division in the Australian Department of Health, Disability and Ageing. The funders did not have any role in the study design, data collection, analysis or interpretation, or reporting. The funders reviewed the manuscript prior to public release.
Bette Liu receives funding from the National Health and Medical Research Council (NHMRC) and the Medical Research Future Fund (paid to the Sydney Children’s Hospital Network and University of New South Wales) and has had travel expenses for presentations covered by the Immunisation Coalition; she was a member of the Australian Technical Advisory Group on Immunisation (ATAGI) during 2019–2023; since September 2024 she has been a member of the World Health Organization Technical Advisory Group on COVID‐19 Vaccine Composition. Kristine Macartney is the Director of the National Centre for Immunisation Research and Surveillance (NCIRS). NCIRS receives funding from the Australian Department of Health, Disability and Ageing, state and territory health departments, the World Health Organization, the Gavi the Vaccine alliance, the Wellcome Trust, and the NHMRC. Kristine Macartney has received travel expenses from these sources to present at vaccination meetings and is a member of the ATAGI, the Advisory Committee on Vaccines, and the WHO Global Advisory Committee on Vaccine Safety (GACVS), among other peak committees.
- 1. World Health Organization. Statement on the antigen composition of COVID‐19 vaccines. 13 Dec 2023. https://www.who.int/news/item/13‐12‐2023‐statement‐on‐the‐antigen‐composition‐of‐covid‐19‐vaccines (viewed Aug 2024).
- 2. Australian Department of Health, Disability, and Ageing. ATAGI recommendations on use of the Moderna and Pfizer monovalent Omicron XBB.1.5 COVID‐19 vaccines [media release]. 20 Nov 2023. https://www.health.gov.au/news/atagi‐recommendations‐on‐use‐of‐the‐moderna‐and‐pfizer‐monovalent‐omicron‐xbb15‐covid‐19‐vaccines (viewed June 2024).
- 3. Link‐Gelles R, Ciesla AA, Mak J, et al. Early estimates of updated 2023–2024 (monovalent XBB.1.5) COVID‐19 vaccine effectiveness against symptomatic SARS‐CoV‐2 infection attributable to co‐circulating Omicron variants among immunocompetent adults: increasing community access to testing program, United States, September 2023 – January 2024. MMWR Morb Mortal Wkly Rep 2024; 73: 77‐83.
- 4. Hansen CH, Moustsen‐Helms IR, Rasmussen M, et al. Short‐term effectiveness of the XBB.1.5 updated COVID‐19 vaccine against hospitalisation in Denmark: a national cohort study. Lancet Infect Dis 2024; 24: e73‐e74.
- 5. Viral Respiratory Diseases Epidemiology and Surveillance Team. COVID‐19 Australia: epidemiology report 85. Reporting period ending 10 March 2024. Commun Dis Intell (2018) 2024; 48.
- 6. Lin DY, Du Y, Xu Y, et al. Durability of XBB.1.5 vaccines against Omicron subvariants. N Engl J Med 2024; 390: 2124‐2127.
- 7. Kirsebom FCM, Stowe J, Lopez Bernal J, et al. Effectiveness of autumn 2023 COVID‐19 vaccination and residual protection of prior doses against hospitalisation in England, estimated using a test‐negative case–control study. J Infect 2024; 89: 106177.
- 8. Andersson NW, Thiesson EM, Pihlström N, et al. Comparative effectiveness of monovalent XBB.1.5 containing covid‐19 mRNA vaccines in Denmark, Finland, and Sweden: target trial emulation based on registry data. BMJ 2024; 3: e001074.
- 9. Monge S, Humphreys J, Nicolay N, et al; VEBIS‐EHR Working Group. Effectiveness of XBB.1.5 monovalent COVID‐19 vaccines during a period of XBB.1.5 dominance in EU/EEA countries, October to November 2023: a VEBIS‐EHR Network study. Influenza Other Respir Viruses 2024; 18: e13292.
- 10. Australian Department of Health, Disability, and Ageing [media release]. ATAGI update on the COVID‐19 vaccination program. 1 Sept 2023. https://www.health.gov.au/news/atagi‐update‐on‐the‐covid‐19‐vaccination‐program (viewed June 2024).
- 11. Liu B, Stepien S, Dobbins T, et al. Effectiveness of COVID‐19 vaccination against COVID‐19 specific and all‐cause mortality in older Australians: a population based study. Lancet Reg Health West Pac 2023; 40: 100928
- 12. Australian Bureau of Statistics. Person level integrated data asset (PLIDA). Undated. https://www.abs.gov.au/about/data‐services/data‐integration/integrated‐data/person‐level‐integrated‐data‐asset‐plida (viewed June 2024).
- 13. Australian Department of Health and Aged Care. COVID‐19 vaccine rollout. 10 Jan 2024. https://www.health.gov.au/sites/default/files/2024‐01/covid‐19‐vaccine‐rollout‐update‐12‐january‐2024.pdf (viewed July 2025).
- 14. Pratt NL, Kerr M, Barratt JD, et al. The validity of the Rx‐Risk Comorbidity Index using medicines mapped to the Anatomical Therapeutic Chemical (ATC) classification system. BMJ Open 2018; 8: e021122.
- 15. Nguyen J, Mitratza M, Volkman H, et al. Effectiveness of the BNT162b2 XBB.1.5‐adapted vaccine against COVID‐19 hospitalization related to the JN.1 variant in Europe: a test‐negative case‐control study using the id.DRIVE platform. EClinicalMedicine 2025; 79: 102995.
- 16. World Health Organization. Statement on the antigen composition of COVID‐19 vaccines. 15 May 2025. https://www.who.int/news/item/15‐05‐2025‐statement‐on‐the‐antigen‐composition‐of‐covid‐19‐vaccines (viewed July 2025).






Abstract
Objectives: To assess the effectiveness of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) monovalent XBB.1.5 variant vaccine for reducing coronavirus disease 2019 (COVID‐19) mortality among people aged 65 years or older.
Study design: Retrospective observational cohort study; analysis of linked 2021 Australian census, Australian Immunisation Register, and death registrations data.
Setting: Australia, 1 August 2023 to 29 February 2024; dominant SARS‐CoV‐2 Omicron subvariants: XBB‐related until early December 2023, then the BA.2.86‐related JN.1.
Participants: People aged 65 years or older on 1 August 2023.
Main outcome measures: Relative vaccine effectiveness by time since most recent booster and booster type (XBB.1.5 variant or other), adjusted for age, gender, state/territory, household income, number of medical conditions, number of general practice visits, and influenza vaccination during 2022.
Results: By 29 February 2024, 1620 COVID‐19‐specific deaths among 4.12 million people aged 65 years or older had been recorded. COVID‐19 mortality was lower among people who had received XBB.1.5 COVID‐19 booster doses during the preceding 90 days (21 [95% confidence interval {CI}, 9–52] per 100 000 person‐years) than among those whose most recent booster had been more than 365 days ago (72 [95% CI, 61–84] per 100 000 person‐years). The relative vaccine effectiveness for XBB.1.5 boosters during the preceding 90 days (v any booster > 365 days) was 74.7% (95% CI, 59.9–84.1%); for other booster types it was 51.6% (95% CI, 39.3–61.4%). Relative vaccine effectiveness declined with time: for any booster during the preceding 91–180 days (v any booster > 365 days) it was 31.2% (95% CI, 18.9–41.6%); for any booster during the preceding 181–365 days it was 13.1% (95% CI, 1.8–23.2%). Relative XBB.1.5 vaccine effectiveness was similar in analyses restricted to 1 December 2023 – 29 February 2024, when the dominant Omicron subvariant was JN.1.
Conclusions: Recent booster vaccination with the XBB.1.5 monovalent COVID‐19 vaccine was highly effective for preventing COVID‐19 deaths among people aged 65 years or older, including during the period in which the JN.1 was the dominant SARS‐CoV‐2 Omicron subvariant. Our findings provide support for the recommendation that people aged 65 years or older receive COVID‐19 vaccine booster doses every six months.