The objective of this consensus statement summary is to provide evidence‐based recommendations for health care professionals in primary care regarding the assessment of metabolic dysfunction‐associated fatty liver disease (MAFLD) in adults. The application of these recommendations will aid in the determination of liver disease severity and assessment of underlying co‐existing conditions, thereby guiding referral pathways for specialist care and monitoring strategies. Key clinical areas covered include: (i) screening and diagnosis, (ii) assessment of extra‐hepatic co‐morbid conditions, (iii) assessment of underlying liver disease, and (iv) monitoring over time. The recommendations are summarised in Box 1 with the complete consensus statement1 available at https://www.gesa.org.au/resources/clinical‐practice‐resources/metabolic‐dysfunction‐associated‐fatty‐liver‐disease‐mafld‐consensus‐statement/.
Methods
This consensus statement summary was developed by applying the principles outlined by the Appraisal of Guidelines for Research and Evaluation (AGREE) II instrument2 and was led by experts in hepatology, general practice, endocrinology, cardiometabolic medicine, chemical pathology, nursing, implementation science and public health, with review by consumer representatives. Recommendation development was supported by a systematic literature search and appraisal using AMSTAR3 and AGREE‐II tools, where appropriate.2 Three rounds of recommendations were circulated and a modified Delphi approach was used to reach consensus, which was defined using an a priori super‐majority of more than 80%.4,5
Levels of evidence for the recommendations were assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system6 with the quality or certainty of evidence classified as high (A), moderate (B), low (C) or very low (D) and the strength of recommendations classified as strong2 or weak.3
Recommendations
MAFLD, formerly known as non‐alcoholic fatty liver disease or NAFLD, is defined by the presence of hepatic steatosis (documented on imaging, biomarker test results or liver histology) with metabolic risk factors including overweight/obesity, type 2 diabetes or two or more features of the metabolic syndrome, such as hypertension, hypertriglyceridemia or low serum high‐density lipoprotein cholesterol levels (Box 2).7 Up to 30% of individuals with MAFLD will have liver inflammation and hepatocellular damage, with or without fibrosis, known as metabolic dysfunction‐associated steatohepatitis (MASH).
MAFLD is the most prevalent condition affecting the liver with an estimated prevalence in Australia and globally of about 30%.8 MAFLD is an increasingly frequent cause of cirrhosis and hepatocellular carcinoma (HCC), and liver‐related deaths due to MAFLD are estimated to increase by 85% in Australia over the coming decade.9 The underlying pathogenesis of MAFLD relates to metabolic dysfunction, and thus the prevalence in people with type 2 diabetes is 55–60%,10,11,12 with a similar prevalence of 55–75% in people with obesity.11,13 MAFLD may also occur in the presence of other metabolic abnormalities among individuals with normal weight or overweight (Box 3).7
Screening and diagnosis
Screening for MAFLD. MAFLD fulfills the majority of criteria required to consider screening in primary care; MAFLD is an important public health problem9 that has a well understood natural history14 including timelines to progression and prognostic factors in relation to who is most at risk of adverse outcomes and hence suitable for treatment.15 The diagnosis is made readily by imaging and once recognised, treatment comprises lifestyle modification (diet and exercise), with effective pharmacotherapy options likely to be available in Australia in the near future.16 Screening for MAFLD appears to be cost‐effective in higher risk groups such as individuals with type 2 diabetes; however, data are needed within the Australian context.17,18,19
Diagnostic methods for MAFLD. Liver ultrasound is widely available, relatively inexpensive and accurate in the detection of hepatic steatosis with meta‐analysis data demonstrating an area under the receiver operating characteristic curve (AUC), sensitivity and specificity for the detection of fatty liver of 0.87, 82% and 87%.20 However, liver ultrasound has its limitations including a degree of operator dependency in test performance, and reduced sensitivity in individuals with obesity or mild hepatic steatosis.
Co‐existing conditions in people with MAFLD
Co‐existing metabolic conditions in MAFLD. MAFLD is associated with several metabolic conditions, including obesity, type 2 diabetes, dyslipidaemia and hypertension. Owing to shared risk factors and pathogenic mechanisms, MAFLD is also associated with increased prevalence and incidence of cardiovascular disease (CVD), obstructive sleep apnoea (OSA) and chronic kidney disease (CKD). Given the increased occurrence of these co‐existing conditions, it is recommended that they are routinely assessed in patients with MAFLD.
Obesity is present in about half of patients diagnosed with MAFLD, and in ~82% of patients with MASH.21 The recommended assessment and management of obesity in people with MAFLD aligns with the Australian Obesity Management Algorithm.22
Up to one‐quarter of patients with MAFLD have type 2 diabetes,23 with the prevalence increasing with the severity of underlying liver histology, being 22% in patients with MAFLD and 44% in patients with MASH.21 Type 2 diabetes is a strong risk factor for the development of hepatic fibrosis (adjusted odds ratio, 2.57) with a two‐ to threefold increased risk of developing liver decompensation and HCC.24,25,26 Screening for type 2 diabetes should be performed according to the Australian Diabetes screening guideline using fasting blood glucose or glycated haemoglobin (HbA1c) levels (https://www.health.gov.au/resources/apps‐and‐tools/the‐australian‐type‐2‐diabetes‐risk‐assessment‐tool‐ausdrisk).
In large meta‐analyses, both hypertension and dyslipidaemia are present in 40–50% of patients with MAFLD.21,23,27,28 Within the Australian context, the prevalence of hypertension was 37% in 626 patients with MAFLD referred from primary care to a tertiary hepatology clinic.29
Risk of CVD morbidity and mortality in MAFLD. CVD is the most common cause of death in people with MAFLD, being responsible for one‐quarter of all deaths.30 A meta‐analysis of seven cohort studies comprising over 13 million individuals, found MAFLD to be associated with a 50% higher risk of fatal or non‐fatal CVD events independent of traditional CVD risk factors.31 MAFLD has also been associated with an increased risk of non‐atherosclerotic CVD including cardiac arrhythmias, structural heart disease and heart failure.32,33,34 Assessment and monitoring for CVD risk should be performed according to recent Australian guidelines.35 Statins are safe in patients with MAFLD, including compensated cirrhosis and thus, when indicated, should not be avoided in people with MAFLD.36
CKD in people with MAFLD. CKD, CVD and metabolic dysfunction (which includes MAFLD) share many pathophysiological features, which can be conceptualised as the cardiovascular–kidney–metabolic syndrome.37 CKD is present in up to one in four individuals with MAFLD23 with a meta‐analysis of 1.2 million individuals with MAFLD demonstrating a 43% increased risk of incident CKD over ten years.38 A study from the UK Biobank has demonstrated that MAFLD is associated with a doubling of risk of end‐stage kidney disease.39 Screening for CKD among people with MAFLD should be performed as directed by Kidney Health Australia guidelines.40
Obstructive sleep apnoea in people with MAFLD. MAFLD is associated with a 6.8 fold increased odds of OSA, which is in part mediated by obesity, with a prevalence of 32% in people with MAFLD in one Australian study.41,42 Although clinical trials have not demonstrated that treatment of OSA improves hepatic steatosis or serum liver enzyme levels,43 the high prevalence of OSA, coupled with impairments in quality of life and a defined treatment strategy warrants assessment with tools such as the STOP‐Bang questionnaire.44
Extra‐hepatic cancer in people with MAFLD. Non‐liver‐related malignancy is the second most common cause of death in people with MAFLD.45 The risk of dying from extra‐hepatic cancer is more than twofold higher than age, sex and region matched population controls with several meta‐analyses demonstrating an increased risk of gastrointestinal, breast, lung, thyroid and genitourinary cancer.46 It is not clear if this relationship is independent of other cancer risk factors such as diabetes; however, participation in population‐based cancer screening (eg, bowel, breast, cervical cancer) should be encouraged in people with MAFLD.
Assessment of liver disease
Assessment of other causes of hepatic steatosis. Additional causes of hepatic steatosis, including excess alcohol consumption and certain medications, should be considered in people with MAFLD due to their different prognoses and treatments. The common causes of a fatty liver are overweight/obesity, type 2 diabetes, alcohol, certain medications (including corticosteroids, methotrexate, anti‐psychotics, valproate, amiodarone, tamoxifen) and hepatitis C (genotype 3). Corticosteroids cause weight gain and insulin resistance, with resultant hepatic steatosis and steatohepatitis; however, advanced fibrosis or cirrhosis appear rare. Chronic methotrexate and amiodarone use have rarely been associated with steatohepatitis and cirrhosis and should be carefully evaluated in patients with hepatic steatosis.47 Genotype 3 hepatitis C virus (HCV) infection interferes with hepatic lipid metabolism and should be considered in people with risk factors for infection and, depending on history, rare disorders such as Wilson disease may also be considered.
Alcohol use in MAFLD. About 5% of Australians drink alcohol daily,48 with alcohol‐related fatty liver disease developing in 90% of people who drink more than 40 g of alcohol per day over a sustained period.49 When combined with excess alcohol consumption, MAFLD increases the risk of alcohol‐related hepatitis and cirrhosis. Moderate alcohol consumption (between 5–30 g per day) may not be sufficient to cause hepatic steatosis; however, may increase the risk of liver fibrosis,50 particularly in the context of metabolic dysfunction, and thus alcohol consumption should be assessed in people presenting with fatty liver.51,52,53
Current National Health and Medical Research Council Australian guidelines recommend that men and women should drink no more than ten standard drinks a week and no more than four standard drinks on any one day; however, it is recognised that the risk of harm is lowered when less alcohol is consumed.54 Patients with cirrhosis should abstain from alcohol due to the increased risk of HCC and decompensation.55
Assessment of other causes of liver disease. Chronic hepatitis B and chronic hepatitis C are present in 0.8% and 0.3% of the Australian population.56,57 An elevated serum alanine aminotransferase (ALT) level (> 40 U/L for men and 35 U/L for women)58 should trigger assessment of risk factors for chronic hepatitis B and chronic hepatitis C, noting that acute intercurrent illness and co‐morbidities may affect liver enzyme levels. Screening for viral hepatitis B and C should be performed with hepatitis B serological testing and HCV antibody (with reflex testing for HCV RNA).
Elevated serum ferritin levels (> 200 ng/ml in women and > 300 ng/ml in men) are present in up to one‐third of people with MAFLD and are more reflective of underlying hepatic steatosis than significant iron load.59,60 Elevated transferrin saturation (> 45%) is present in less than 10% of MAFLD patients but should precipitate screening for genetic haemochromatosis. Phlebotomy is not indicated in patients with hyperferritinaemia in the absence of genetic haemochromatosis or iron overload (ie, normal transferrin saturation levels) as it does not improve the underlying metabolic dysfunction or liver injury.61,62
Assessment of liver fibrosis. Liver fibrosis can be quantified by liver biopsy and a staging score that categorises the severity against a spectrum from zero (no fibrosis) to four (equivalent to cirrhosis).63 Advanced fibrosis (ie, stage 3 and 4) is present in about 5%64,65,66 of patients with MAFLD and predicts an increased risk of future liver decompensation, HCC and liver‐related mortality.15 Early identification of people with advanced liver fibrosis using non‐invasive tests (NITs) provides the opportunity for point‐of‐care prognostication, determination of clinical management priorities, determination of need for specialist referral or additional investigations and intervention to reduce disease progression.
To predict fibrosis risk, assessment of liver fibrosis requires blood‐based NITs and/or elastography. Importantly, standard liver tests, including for bilirubin, aminotransferases and albumin, are not accurate in detecting advanced liver fibrosis and may even show normal results in the presence of cirrhosis.67,68 Similarly, ultrasound and computed tomography are inaccurate for identifying advanced fibrosis and lack sensitivity for determining cirrhosis.69
First‐line testing for liver fibrosis. The Fibrosis‐4 Index (FIB‐4) uses common laboratory results to derive a predictive algorithm consisting of age [years] × AST [U/L]/(platelet count [109/L] × √ALT [U/L]). FIB‐4 can easily be accessed via online calculators and has been broadly validated as an accurate predictor of advanced fibrosis in people with MAFLD with a meta‐analysis of 37 studies involving 5735 individuals finding a summary AUC statistic of 0.76.70 The Royal College of Pathologists of Australasia has endorsed the uniform reporting of FIB‐4 by pathology laboratories across Australia.
FIB‐4 scores below a threshold of 1.3 exclude advanced liver fibrosis with a negative predictive value (NPV) of 95–97% in primary care and sensitivity of 74% (95% confidence interval [CI], 72–76%). Scores above 2.67 are 94% specific (95% CI, 93–94%) for advanced fibrosis but only have a positive predictive value of 24–40% in primary care settings, demonstrating the need for referral for specialist review and further confirmatory testing.70 FIB‐4 scores between 1.3 and 2.67 are indeterminate and these patients should undergo second‐line fibrosis testing.
Similar to other biochemical assays, the FIB‐4 test has analytical variation between laboratories. Testing of aspartate aminotransferase (AST) and ALT levels by the Royal College of Pathologists of Australasia Quality Assurance Program in over 160 Australian laboratories has demonstrated the total analytical coefficient of variation for FIB‐4 scores across Australia to be between 8 and 11% (personal communication, Professor Graham Jones, SydPath, St Vincents Hospital, University of New South Wales, June 2025) when age and platelet values are fixed. Thus, for simplicity, it is reasonable in clinical practice to round the upper threshold of FIB‐4 scores from 2.67 to 2.7.
FIB‐4 is inaccurate in individuals younger than 35 years of age71 and should not be used in this population, although the risk of advanced liver fibrosis in young adults is very low. The specificity of FIB‐4 reduces with increasing age, such that a threshold of 2.0 instead of 1.3 should be used in patients older than 65 years to exclude advanced fibrosis.71 FIB‐4 may be falsely elevated in patients with thrombocytopaenia of non‐hepatic aetiology (eg, immune thrombocytopaenic purpura or harmful alcohol use) or in cases of acute hepatic injury from any cause or acute muscle injury, which may increase AST levels.
Second‐line testing for liver fibrosis. About 20% of individuals with MAFLD will have an indeterminate FIB‐4 score (between 1.3 and 2.7)72 and will require a second‐line test to determine the risk of advanced fibrosis and future liver‐related morbidity and mortality (Box 4). Liver elastography (including vibration controlled transient elastography or Fibroscan, and shearwave elastography [SWE]) or a direct serum fibrosis test (including Hepascore or Enhanced Liver Fibrosis [ELF] test) are recommended in this patient group owing to their higher accuracy compared to FIB‐4.
The liver stiffness measurement (LSM) from Fibroscan or SWE correlates with fibrosis severity and predicts the likelihood of advanced liver fibrosis. Fibroscan has been extensively validated with a LSM threshold of less than 8.0 kPa excluding advanced fibrosis with 86% sensitivity and an NPV of 98–99%.68 Patients with MAFLD and a FIB‐4 score between 1.3 and 2.67 and Fibroscan LSM less than 8.0 kPa are at a low risk of liver‐related morbidity and can be managed in primary care.73
SWE is less validated in MAFLD compared with Fibroscan; however, a meta‐analysis of 1209 patients found reasonable accuracy (AUC, 0.72–0.89), sensitivity (72–80%) and specificity (72–86%) for predicting advanced liver fibrosis,74 and a prospective comparative study found equivalent accuracy between Fibroscan and SWE.75 Similarly, Fibroscan and SWE, using the same threshold of 8 kPa, have equivalent accuracy for diagnosing advanced fibrosis among patients with a FIB‐4 score more than 1.3.76
LSM may be falsely elevated in acute hepatitis, cholestasis, liver congestion (eg, right heart failure), non‐fasting states or focal liver lesions and should be interpreted with caution in these patient groups.77 Elastography is less accurate with increasing body mass index (BMI) or in individuals with a skin to liver capsule distance greater than 30 mm, and thus reliability criteria should be included in elastography reports.78,79 Unreliable elastograms should prompt assessment by an alternative second‐line NIT (ELF or Hepascore) or referral to a clinician with expertise in liver disease. There is limited availability of Fibroscan devices in Australia, with the majority in major metropolitan hospital centres, whereas SWE is increasingly available through public and private radiology facilities. The lack of Medicare rebate for any of the second‐line tests provides a disincentive due to cost to the patient and/or provider.
Direct serum fibrosis tests incorporate serum markers of fibrogenesis or fibrinolysis and have greater accuracy than FIB‐4 for the prediction of advanced liver fibrosis.80,81 Two tests are currently available in Australia: Hepascore and ELF.
A meta‐analysis of 11 studies (4452 patients with MAFLD) showed that ELF has good accuracy for predicting advanced liver fibrosis with a summary AUC value of 0.83 (95% CI, 0.71–0.90).82 When using a threshold of 9.8, the sensitivity and specificity for advanced liver fibrosis was 65% and 86%, with a positive predictive value of 34% and NPV of 96%.82 Therefore, an ELF result of 9.8 is recommended as the threshold for referral for specialist review in patients with an indeterminate FIB‐4 score (ie, between 1.3 and 2.7). When introduced into primary care practices in the United Kingdom, sequential use of FIB‐4 followed by ELF led to a fourfold increase in the diagnosis of advanced fibrosis and cirrhosis and an 81% reduction in unnecessary referrals.83
Hepascore is an algorithm developed in Australia that has good accuracy for the prediction of advanced fibrosis and long term risks for liver‐related death, decompensation and HCC in patients with MAFLD.29,84,85 Hepascore had similar accuracy to Fibroscan for predicting advanced fibrosis (AUC, 0.88 v 0.80) in an Australian cohort of 271 patients with MAFLD, with a threshold of 0.60 having a sensitivity of 64% and specificity of 93%.85 The sequential use of FIB‐4 followed by Hepascore for indeterminate FIB‐4 scores, provided 80% diagnostic accuracy and 100% specificity; however, only 50% sensitivity for the diagnosis of advanced fibrosis in a study of 938 MAFLD patients.86 People with MAFLD referred from primary care for specialist review who have a Hepascore less than 0.60 have an NPV of 97–100% for future liver decompensation or HCC in the next ten years, suggesting that individuals below this threshold can be monitored in primary care.29
Clinicians using direct serum fibrosis tests should be aware of the potential for falsely positive tests in cases of acute hepatitis, or with haemolysis or Gilbert syndrome in the case of Hepascore, which includes bilirubin as an analyte. Serum fibrosis tests are potentially more accessible to patients in regional and remote settings than elastography. The costs of direct serum fibrosis tests are not reimbursed by Medicare and the cost to the patient remains a significant barrier towards widespread adoption. Other direct serum fibrosis tests have been validated internationally but are not available in Australia.
People with MAFLD‐related cirrhosis. People with MAFLD can progress to cirrhosis in the absence of significant symptoms or clinical signs.87 The development of jaundice, ascites, hepatic encephalopathy or gastro‐oesophageal varices indicates significant liver dysfunction or portal hypertension and heralds a significantly shortened life expectancy.55 Laboratory results indicative of advanced liver disease include hyperbilirubinemia, synthetic dysfunction (hypoalbuminemia, elevated INR [international normalised ratio]) and portal hypertension (thrombocytopaenia) may precede clinical deterioration and should initiate prompt referral to a specialist in liver disease. Imaging features of cirrhosis (nodular liver surface) in association with portal hypertension (splenomegaly, portosystemic collaterals, ascites) have a more than 90% specificity for a diagnosis of cirrhosis and should also prompt referral.69
Monitoring for progression of liver disease
Fibrosis progression in people with MAFLD. Patients with low NIT scores may develop progressive liver fibrosis, which is associated with an increased risk of future liver‐related morbidity and mortality.88 Overall, the progression of liver fibrosis among people with MAFLD is relatively slow with the average time to progress one fibrosis stage in individuals with no or minimal fibrosis (stage 0 or 1) being ten years.14 Nonetheless, some patients will progress relatively quickly with 6–15% of people with stage 0 or stage 1 fibrosis progressing to advanced fibrosis or cirrhosis (stage 3 or 4) over five years.14
Using NITs to monitor liver fibrosis progression. Patients with low FIB‐4 scores (< 1.3) should be monitored with a repeat test at least every three years. About 20% of patients increase from low to intermediate or high risk thresholds over three years, signalling an increased risk of future cirrhosis, HCC and liver‐related death.89,90 Among a cohort of 202 319 patients with MAFLD from the United States, the incidence of cirrhosis or HCC in patients with a persistently low FIB‐4 score was 0.4 cases per 1000/year, which increased to 1.3 cases per 1000/year in individuals transitioning from low to indeterminate risk, and was highest in patients transitioning from low to high risk, at 8.6 cases per 1000/year.89 Caution is required when interpreting small changes in FIB‐4 scores over time as there is modest within‐person variation (within‐subject coefficient of variation, ~13%) (personal communication, Professor Graham Jones, SydPath, St Vincents Hospital, University of New South Wales, Sydney, June 2025).
Fibrosis progression is more likely with increasing liver enzyme elevations and type 2 diabetes, especially when glycaemic control is poorly managed; a 10 unit increase in AST (but not ALT) is associated with a 30% increased risk of fibrosis progression whereas a 1% increase in HbA1c level is associated with a 15% higher chance of an increase in fibrosis stage.91,92 In addition, patients with type 2 diabetes are 69% more likely to have progressive fibrosis compared with patients without type 2 diabetes.93 Thus repeat FIB‐4 testing may be considered at shorter time intervals (eg, every 1–2 years) in patients with type 2 diabetes, rising AST or elevated HbA1c levels.
Monitoring people with MAFLD that are older than 75 years. People aged over 75 years with MAFLD but without advanced liver fibrosis do not appear to have an increased risk of mortality and have a very low risk of developing cirrhosis and its complications.94,95 In contrast, older patients with cirrhosis have a significantly increased risk of incident HCC and liver‐related death.96 The decision to screen and monitor for fibrosis progression in these individuals needs to be weighed based on the competing risks of co‐existing health conditions.
Surveillance for HCC in people with MAFLD. Patients with cirrhosis related to MAFLD are at a significantly increased risk of developing HCC, with an annual rate of over 3.5%97 leading to the recommendation for surveillance by current Australian guidelines.98 In contrast, the risk of HCC among people with MAFLD but without cirrhosis is very low (< 0.05%/year) meaning that surveillance is impractical in people with MAFLD but without cirrhosis.97,99
HCC surveillance is performed with liver‐directed ultrasound (providing there is good visualisation of the liver) with or without serum α‐fetoprotein levels every six months and should be coordinated in conjunction with a specialist with expertise in liver disease. Early detection of small HCCs by surveillance enables curative therapies and improved survival.100,101 HCC surveillance should be limited to people of Child‐Pugh A or B status or Child‐Pugh C people who are potential liver transplant candidates, and those without life‐limiting co‐morbidities and reasonable functional status.102
Monitoring of co‐morbid conditions
Impact of weight change on liver histology and outcomes in MAFLD. Weight gain and obesity are intimately associated with the development of MAFLD and conversely, weight loss and the attendant improvement in metabolic dysfunction are associated with clinical benefit.16 Relatively small amounts of weight loss can improve liver histology with a 5 kg reduction in weight associated with a 39% probability of MASH resolution and 31% improvement in liver fibrosis over 1.5–2 years.103 Weight loss related to bariatric surgery in patients with MAFLD and obesity is associated with a reduction in major adverse cardiovascular and liver‐related events (including development of cirrhosis, HCC and liver‐related death).104 Conversely, weight gain is associated with a lower odds of improvement in MASH and fibrosis.103 Monitoring of weight, BMI or waist circumference provides an insight into the likelihood of disease progression or regression and should precipitate further assessment and management according to the Australian Obesity Management Algorithm in the presence of ongoing weight gain.
Incidence of type 2 diabetes in people with MAFLD. A diagnosis of MAFLD often precedes the development of type 2 diabetes and the presence of MAFLD is associated with a two‐ to threefold increased risk of incident type 2 diabetes.105,106 MAFLD may promote the development of type 2 diabetes with hepatic steatosis promoting hepatic insulin resistance and increased gluconeogenesis.107 The overall incidence rate of type 2 diabetes in people with underlying MAFLD is estimated at 2.7% (95% CI, 0.7–4.4%) per year with hypertriglyceridemia, pre‐diabetes and low levels of physical activity increasing the risk.108,109 The onset of type 2 diabetes in people with MAFLD heralds an increased likelihood of liver‐related complications with a twofold higher risk of hepatic decompensation and a fivefold increased risk of future HCC.24 Due to the increased risk of incident type 2 diabetes and associated ramifications on patient outcomes, it is recommended that people with MAFLD be periodically screened for the development of type 2 diabetes as per current Australian guidelines.
Conclusion
The prevalence of MAFLD and associated end‐stage liver disease are predicted to increase significantly in Australia in the coming decade.9 To assist in the assessment and management of people with MAFLD, this consensus statement summary has been developed following a systematic literature review and broad input from a diverse range of stakeholders and experts. It provides a structured evidence‐based framework to aid health professionals working in primary care in the identification and assessment of MAFLD to aid appropriate referral. This in turn allows the specialist to focus on investigation and management including liver‐directed pharmacotherapy and surveillance for liver‐related complications such as HCC and gastro‐oesophageal varices. Ideally, this will improve the efficiency and workflow for health care practitioners in primary care when reviewing people with MAFLD. Ultimately, the implementation of the recommendations within this consensus statement summary seeks to improve the quality of life and reduce the burden of disease in MAFLD patients.
Box 1 – Summary of recommendations
|
|
|||||||||||||||
|
Recommendations |
|||||||||||||||
|
Who should be assessed for metabolic dysfunction‐associated fatty liver disease (MAFLD)? |
|||||||||||||||
|
1. Adults with obesity and/or type 2 diabetes, or two or more metabolic risk factors* should be assessed for MAFLD |
|||||||||||||||
|
Evidence quality: low; grade of recommendation: strong |
|||||||||||||||
|
How should MAFLD be diagnosed? |
|||||||||||||||
|
2. Liver ultrasound should be the first‐line test to diagnose hepatic steatosis in people at high risk of MAFLD |
|||||||||||||||
|
Evidence quality: moderate; grade of recommendation: strong |
|||||||||||||||
|
What co‐morbid conditions should be assessed in people with MAFLD? |
|||||||||||||||
|
3. People with obesity and MAFLD should be assessed in accordance with the Australian Obesity Management Algorithm |
|||||||||||||||
|
Evidence quality: low; grade of recommendation: strong |
|||||||||||||||
|
4. People with MAFLD should be assessed for undiagnosed type 2 diabetes using measurement of fasting blood glucose or HbA1c levels |
|||||||||||||||
|
Evidence quality: moderate; grade of recommendation: strong |
|||||||||||||||
|
5. People with MAFLD should be assessed and monitored for the presence and risk of future cardiovascular disease according to current Australian guidelines |
|||||||||||||||
|
Evidence quality: high; grade of recommendation: strong |
|||||||||||||||
|
6. Baseline assessment for potential co‐morbid conditions of chronic kidney disease and obstructive sleep apnoea should be considered for people with MAFLD |
|||||||||||||||
|
Evidence quality: moderate; grade of recommendation: weak |
|||||||||||||||
|
How should other aetiologies of liver disease be assessed in people with MAFLD? |
|||||||||||||||
|
7. People with MAFLD should be assessed for other common causes of fatty liver and liver disease |
|||||||||||||||
|
Evidence quality: low; grade of recommendation: strong |
|||||||||||||||
|
8. People with MAFLD should undergo screening for harmful alcohol use |
|||||||||||||||
|
Evidence quality: moderate; grade of recommendation: strong |
|||||||||||||||
|
9. People with MAFLD and elevated serum aminotransferase levels should undergo baseline evaluation for hepatitis B and C infection |
|||||||||||||||
|
Evidence quality: moderate. grade of recommendation strong |
|||||||||||||||
|
10. People with MAFLD and elevated serum aminotransferase levels should undergo evaluation for iron overload |
|||||||||||||||
|
Evidence quality: moderate. grade of recommendation strong |
|||||||||||||||
|
How should the severity of liver disease be assessed in people with MAFLD? |
|||||||||||||||
|
11. Non‐invasive testing should be offered to people with MAFLD to assess their risk of liver fibrosis |
|||||||||||||||
|
Evidence quality: moderate; grade of recommendation: strong |
|||||||||||||||
|
12. A non‐invasive test such as FIB‐4, should be offered as an initial test to help rule out the risk of advanced liver fibrosis among people with MAFLD |
|||||||||||||||
|
Evidence quality: moderate; grade of recommendation: strong |
|||||||||||||||
|
13. A second‐line assessment with liver elastography or a direct liver fibrosis serum test should be performed in people with MAFLD and a FIB‐4 score between 1.3 and 2.7. If these are unavailable, referral to a clinician with expertise in liver disease should be considered |
|||||||||||||||
|
Evidence quality: low; grade of recommendation: strong |
|||||||||||||||
|
14. People with MAFLD and a FIB‐4 score > 2.7 or elevated results of a direct liver fibrosis serum test or liver elastogram, should be referred to a clinician with expertise in liver disease |
|||||||||||||||
|
Evidence quality: low; grade of recommendation: strong |
|||||||||||||||
|
15. People with MAFLD and clinical, laboratory or imaging evidence of cirrhosis should be referred to a clinician with expertise in liver disease |
|||||||||||||||
|
Evidence quality: high; grade of recommendation: strong |
|||||||||||||||
|
How should liver fibrosis in people with MAFLD be monitored over time? |
|||||||||||||||
|
16. People with MAFLD who have an initial non‐invasive fibrosis test showing a low risk of advanced fibrosis are recommended to undergo repeat non‐invasive fibrosis testing in three years |
|||||||||||||||
|
Evidence quality: low; grade of recommendation: strong |
|||||||||||||||
|
17. People with MAFLD and a FIB‐4 score between 1.3 and 2.7 who undergo elastography or a direct liver fibrosis serum test that shows a low risk of advanced liver fibrosis should be offered repeat testing with a FIB‐4 at least every three years |
|||||||||||||||
|
Evidence quality: low; grade of recommendation: weak |
|||||||||||||||
|
18. For people who are 75 years or older and have MAFLD, routine monitoring for fibrosis progression should be performed on a case‐by‐case basis, depending on their co‐morbid conditions and life expectancy |
|||||||||||||||
|
Evidence quality: low; grade of recommendation: strong |
|||||||||||||||
|
19. People with cirrhosis who would be willing and suitable for HCC therapy should be undergoing six‐monthly surveillance for HCC using appropriate imaging with or without serum α‐fetoprotein testing |
|||||||||||||||
|
Evidence quality: low; grade of recommendation: strong |
|||||||||||||||
|
How should co‐morbid conditions be monitored over time in people with MAFLD? |
|||||||||||||||
|
20. Weight, BMI and/or waist circumference should be monitored at least annually in people with MAFLD to guide management |
|||||||||||||||
|
Evidence quality: low; grade of recommendation: strong |
|||||||||||||||
|
21. People with MAFLD should be monitored for the development of type 2 diabetes according to current Australian guidelines |
|||||||||||||||
|
Evidence quality: moderate; grade of recommendation: strong |
|||||||||||||||
|
|
|||||||||||||||
|
BMI = body mass index; FIB‐4 = Fibrosis‐4 Index; HbA1c = glycated haemoglobin; HCC = hepatocellular carcinoma; MAFLD = metabolic dysfunction‐associated fatty liver disease. *Metabolic risk factors: waist circumference ≥ 102 cm in men of European ancestry and ≥ 88 cm in women of European ancestry (or ≥ 90 cm for men and ≥ 80 cm for women in First Nations Australians and Asians); systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg or taking medication for high blood pressure; plasma triglyceride levels ≥ 1.7 mmol/L or taking medication for elevated triglyceride levels; plasma high‐density lipoprotein cholesterol level < 1.0 mmol/L for men and < 1.3 mmol/L for women or taking medication for reduced high‐density lipoprotein cholesterol levels; pre‐diabetes (ie, fasting glucose levels of 6.1–6.9 mmol/L, or 2‐hour post‐load glucose levels of 7.8–11.0 mmol, or HbA1c level of 6.0–6.4%). |
|||||||||||||||
Box 2 – Diagnostic criteria for metabolic dysfunction‐associated fatty liver disease

* Metabolic risk factors include: central obesity, hypertension, dyslipidaemia, pre‐diabetes, insulin resistance (high score on the Homeostatic Model Assessment of Insulin Resistance).
Box 3 – Major metabolic risk factors for metabolic dysfunction‐associated fatty liver disease (MAFLD)
|
Risk factor |
Prevalence of MAFLD 10,27 |
||||||||||||||
|
|
|||||||||||||||
|
Overweight |
30% |
||||||||||||||
|
Obesity |
55–75% |
||||||||||||||
|
Type 2 diabetes |
55–60% |
||||||||||||||
|
Dyslipidaemia |
55% |
||||||||||||||
|
Hypertension |
50% |
||||||||||||||
|
Metabolic syndrome* |
70% |
||||||||||||||
|
|
|||||||||||||||
|
* Metabolic syndrome consists of at least three features of: central obesity, hypertension, pre‐diabetes, low high‐density lipoprotein cholesterol and hypertriglyceridemia. |
|||||||||||||||
Box 4 – Flowchart showing the pathways when assessing a patient with metabolic dysfunction‐associated fatty liver disease (MAFLD)
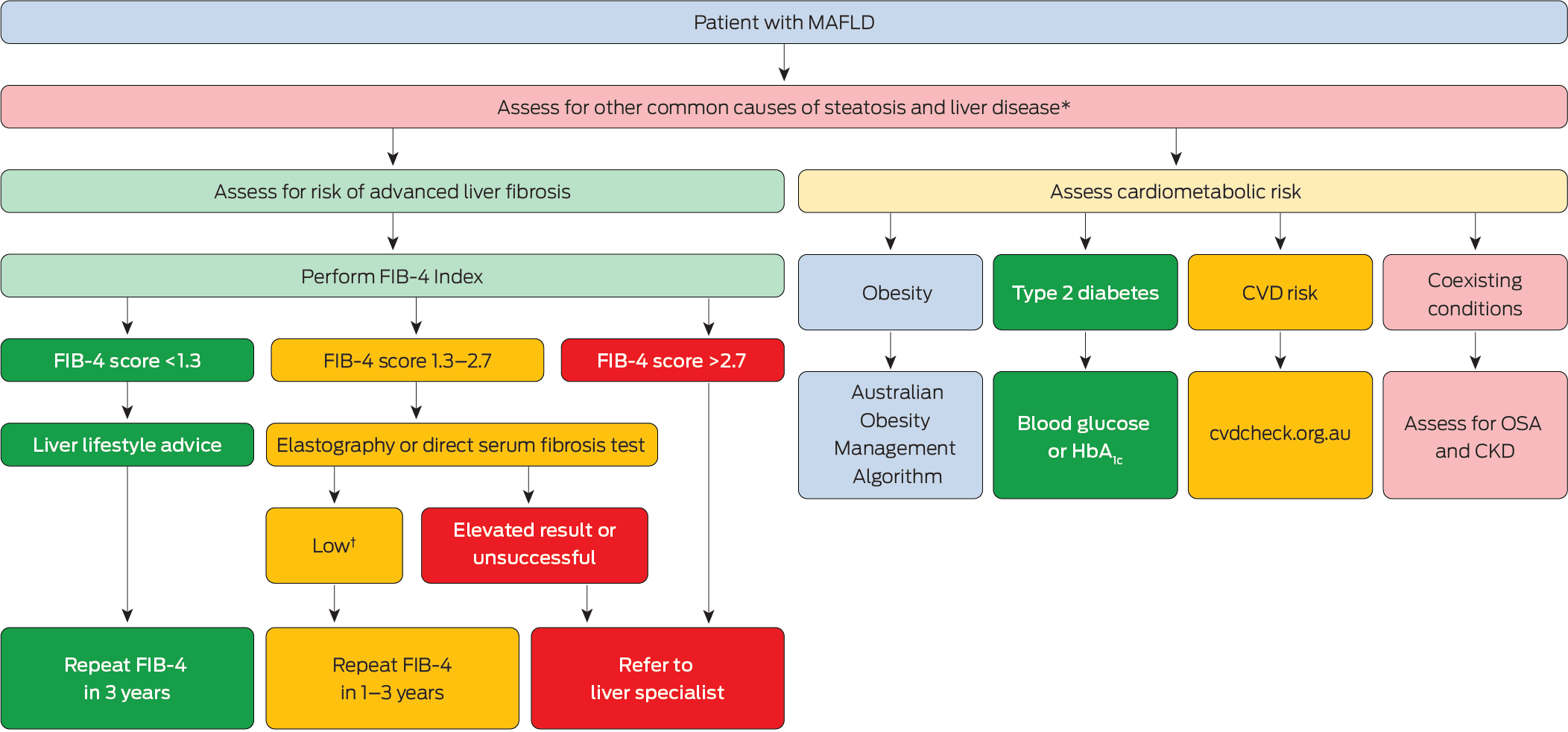
CKD = chronic kidney disease; CVD = cardiovascular disease; FIB‐4 = Fibrosis‐4 Index; HbA1c = glycated haemoglobin; MAFLD = metabolic dysfunction‐associated fatty liver disease; OSA = obstructive sleep apnoea.* Evaluate alcohol intake, medications, risk factors for viral hepatitis and iron overload.† Low thresholds for second‐line fibrosis tests include: vibration controlled transient elastography (8 kPa), shearwave elastography (8 kPa), Hepascore (0.60) and Enhanced Liver Fibrosis test (9.8). Patients with readings above these thresholds should be referred to a specialist in liver disease.
Provenance: Not commissioned; externally peer reviewed.
- Leon A Adams1,2
- William W Kemp3
- Kate R Muller4
- Elizabeth E Powell5,6
- Stuart K Roberts3,7
- Luis Calzadilla Bertot1
- Stephanie Best8
- Gary Deed7,9
- Jon D Emery10
- Samantha L Hocking11,12
- Graham R Jones13,14
- John S Lubel3,7
- Sinead Sheils15
- Stephen M Twigg11,14
- Gerald F Watts16
- Jacob George17
- 1 University of Western Australia, Perth, WA
- 2 Sir Charles Gairdner Hospital, Perth, WA
- 3 Alfred Health, Melbourne, VIC
- 4 Flinders Medical Centre, Adelaide, SA
- 5 Princess Alexandra Hospital, Adelaide, SA
- 6 QIMR Berghofer Medical Research Institute, Brisbane, QLD
- 7 Monash University, Melbourne, VIC
- 8 University of Melbourne, Melbourne, VIC
- 9 HealthcarePlus Medical, Brisbane, QLD
- 10 Centre for Cancer Research, University of Melbourne, Melbourne, VIC
- 11 University of Sydney, Sydney, NSW
- 12 Royal Prince Alfred Hospital, Sydney, NSW
- 13 St Vincent's Hospital, Sydney, NSW
- 14 University of New South Wales, Sydney, NSW
- 15 AW Morrow Gastroenterology and Liver Centre, Royal Prince Alfred Hospital, Sydney, NSW
- 16 Royal Perth Hospital, Perth, WA
- 17 Storr Liver Centre, Westmead Institute of Medical Research, Sydney, NSW
Open access:
Open access publishing facilitated by The University of Western Australia, as part of the Wiley ‐ The University of Western Australia agreement via the Council of Australian University Librarians.
Unrestricted grant funding was provided to the Gastroenterological Society of Australia (GESA) for completion of this consensus statement summary. Details of GESA's funding sources are available on the website (www.gesa.org.au). Editorial independence was maintained throughout the manuscript development. The funders had no input into the study planning, writing or publication and no role in study design, analysis, interpretation or reporting. The following collaborators contributed to the design, participation in the modified Delphi process and in the reviewing of the recommendations: Nicole Allard, Oyekoya Ayonrinde, Shopna Bag, Tim Davis, Anouk Dev, Elif Ekinci, Mohammed Eslam, Charlotte Hespe, Andrew Kirke, Graeme MacDonald, Suzanne Mahady, Avik Majumdar, John Olynyk, Milan Piya, Marno Ryan, Ashim Sinha, Simone Strasser, Alan Wigg, Sue Williams and Amany Zekry. We thank Meike Fruechtl (Project Officer) for her tireless administrative and logistic support and co‐ordination of steering committee and working group members.
Leon Adams has received honoraria for participating on advisory boards and speaker fees during development of the metabolic dysfunction‐associated fatty liver disease (MAFLD) consensus statement summary from Pfizer, Gilead, Roche Diagnostics and Novartis. Mohammed Eslam has received personal fees from Pfizer and honoraria from Sanofi. Jacob George was on advisory boards and receives honoraria for talks from Novo Nordisk, AstraZeneca, Roche, BMS, Pfizer, Cincera, Pharmaxis and Boehringer Mannheim. Samantha Hocking has received honoraria from or participated on advisory boards for Eli Lilly, Novo Nordisk, iNova, Sanofi, AstraZeneca, Servier, Amgen, Nestle Health Sciences, Seqirus, Pfizer and Johnson & Johnson. John Lubel has received speaker fees from Norgine and Gilead, has presented at sponsored GP dinners (no personal fees) for Norgine and Dr Falk Pharma, has received sponsorship for a GP educational event (no speaker fee) from Viatris Pty Ltd, is an IPSEN advisory consultant and has received speaker fees for internal staff education, and received sponsorship from IPSEN to attend the EASL 2024 meeting in Milan, Italy. Norgine partially supported his PhD student to travel to an international conference. Kate Muller has received honoraria for participating on advisory boards and speaker fees during development of the MAFLD consensus statement summary from Chiesi, Eisai, AstraZeneca and Novo Nordisk. Elizabeth Powell has received honoraria for advisory board participation and support for an educational event from Novo Nordisk and has had access to the ELF test provided by Siemens Healthineers. Simone Strasser has received honoraria for participating on advisory boards and speaker fees during development of the MAFLD consensus statement summary from Chiesi, Eisai, Sirtex, Norgine, Roche Products, Roche Diagnostics, AstraZeneca, Otsuka and Pfizer. The University of Western Australia (employer of Leon Adams) holds a US patent for Hepascore. The university does not receive any royalties or benefits from this patent.
Author contribution statement:
Adams LA: Conceptualization, data curation, funding acquisition, methodology, project administration, formal analysis, supervision, writing – original draft, writing – review and editing. Kemp WW: Conceptualization, supervision, writing – original draft, writing – review and editing. Muller KR: Conceptualization, supervision, writing – original draft, writing – review and editing. Powell EE: Conceptualization, supervision, writing – original draft, writing – review and editing. Roberts SK: Conceptualization, supervision, writing – original draft, writing – review and editing. Calzadilla Bertot L: Data curation, formal analysis, writing – original draft, writing – review and editing. Best S: Conceptualization. Deed G: Conceptualization, writing – original draft, writing – review and editing. Emery JD: Conceptualization, writing – original draft, writing – review and editing. Hocking SL: Conceptualization, writing – original draft, writing – review and editing. Jones GR: Conceptualization, writing – original draft, writing – review and editing. Lubel JS: Conceptualization, methodology, writing – original draft, writing – review and editing. Sheils S: Conceptualization, writing – original draft, writing – review and editing. Twigg SM: Conceptualization, writing – original draft, writing – review and editing. Watts GF: Conceptualization, writing – original draft, writing – review and editing. George J: Conceptualization, funding acquisition, methodology, supervision, writing – original draft, writing – review and editing.
- 1. MAFLD Consensus Statement Working Group. Recommendations for the assessment of metabolic dysfunction‐associated fatty liver disease (MAFLD) in primary care: a consensus statement. Melbourne: Gastroenterological Society of Australia, 2024. https://www.gesa.org.au/resources/clinical‐practice‐resources/metabolic‐dysfunction‐associated‐fatty‐liver‐disease‐mafld‐consensus‐statement/
- 2. Brouwers MC, Kho ME, Browman GP, et al for the AGREE Next Steps Consortium. AGREE II instrument. Agree Research Trust, 2017. https://www.agreetrust.org/wp‐content/uploads/2017/12/AGREE‐II‐Users‐Manual‐and‐23‐item‐Instrument‐2009‐Update‐2017.pdf (viewed Sept 2020).
- 3. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ 2017; 358: j4008.
- 4. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995; 311: 376‐380.
- 5. Lubel JS, Roberts SK, Strasser SI, et al. Australian recommendations for the management of hepatocellular carcinoma: a consensus statement. Med J Aust 2021; 214: 475‐483. https://www.mja.com.au/journal/2021/214/10/australian‐recommendations‐management‐hepatocellular‐carcinoma‐consensus
- 6. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924‐926.
- 7. Eslam M, Newsome P, Sarin SK, et al. A new definition for metabolic dysfunction‐associated fatty liver disease: an international expert consensus statement. J Hepatol 2020; 73: 201‐209.
- 8. Chan KE, Koh TJL, Tang ASP, et al. Global prevalence and clinical characteristics of metabolic‐associated fatty liver disease: a meta‐analysis and systematic review of 10 739 607 individuals. J Clin Endocrinol Metab 2022; 107: 2691‐2700.
- 9. Adams LA, Roberts SK, Strasser SI, et al. Nonalcoholic fatty liver disease burden: Australia, 2019‐2030. J Gastroenterol Hepatol 2020; 35: 1628‐1635.
- 10. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hepatol 2019; 71: 793‐801.
- 11. Roberts SK, Majeed A, Glenister K, et al. Prevalence of non‐alcoholic fatty liver disease in regional Victoria: a prospective population‐based study. Med J Aust 2021; 215: 77‐82. https://www.mja.com.au/journal/2021/215/2/prevalence‐non‐alcoholic‐fatty‐liver‐disease‐regional‐victoria‐prospective
- 12. Farrell AM, Magliano DJ, Shaw JE, et al. A problem of proportions: estimates of metabolic associated fatty liver disease and liver fibrosis in Australian adults in the nationwide 2012 AusDiab Study. Sci Rep 2022; 12: 1956.
- 13. Wong VW‐S, Wong GL‐H, Woo J, et al. Impact of the new definition of metabolic associated fatty liver disease on the epidemiology of the disease. Clin Gastro Hepatol 2021; 19: 2161‐2171.
- 14. Le P, Payne JY, Zhang L, et al. Disease state transition probabilities across the spectrum of NAFLD: a systematic review and meta‐analysis of paired biopsy or imaging studies. Clin Gastroenterol Hepatol 2023; 21: 1154‐1168.
- 15. Ng CH, Lim WH, Hui Lim GE, et al. Mortality outcomes by fibrosis stage in nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2023; 21: 931‐939.
- 16. Vilar‐Gomez E, Martinez‐Perez Y, Calzadilla‐Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015; 149: 367‐378.
- 17. Noureddin M, Jones C, Alkhouri N, et al. Screening for nonalcoholic fatty liver disease in persons with type 2 diabetes in the United States is cost‐effective: a comprehensive cost‐utility analysis. Gastroenterology 2020; 159: 1985‐1987.
- 18. Corey KE, Klebanoff MJ, Tramontano AC, et al. Screening for nonalcoholic steatohepatitis in individuals with type 2 diabetes: a cost‐effectiveness analysis. Dig Dis Sci 2016; 61: 2108‐2117.
- 19. Congly SE, Shaheen AA, Swain MG. Modelling the cost effectiveness of nonalcoholic fatty liver disease risk stratification strategies in the community setting. PLoS One 2021; 16: e0251741.
- 20. Ballestri S, Mantovani A, Byrne CD, et al. Diagnostic accuracy of ultrasonography for the detection of hepatic steatosis: an updated meta‐analysis of observational studies. Metab Target Organ Damage 2021; 1: 7.
- 21. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease‐Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73‐84.
- 22. Markovic TP, Proietto J, Dixon JB, et al. The Australian Obesity Management Algorithm: a simple tool to guide the management of obesity in primary care. Obes Res Clin Pract 2022; 16: 353‐363.
- 23. Lim GEH, Tang A, Ng CH, et al. An observational data meta‐analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol 2023; 21: 619‐629.
- 24. Huang DQ, Noureddin N, Ajmera V, et al. Type 2 diabetes, hepatic decompensation, and hepatocellular carcinoma in patients with non‐alcoholic fatty liver disease: an individual participant‐level data meta‐analysis. Lancet Gastroenterol Hepatol 2023; 8: 829‐836.
- 25. Bertot LC, Jeffrey GP, de Boer B, et al. Diabetes impacts prediction of cirrhosis and prognosis by non‐invasive fibrosis models in non‐alcoholic fatty liver disease. Liver Int 2018; 38: 1793‐1802.
- 26. O’Beirne J, Skoien R, Leggett BA, et al. Diabetes mellitus and the progression of non‐alcoholic fatty liver disease to decompensated cirrhosis: a retrospective cohort study. Med J Aust 2023; 219: 358‐365. https://www.mja.com.au/journal/2023/219/8/diabetes‐mellitus‐and‐progression‐non‐alcoholic‐fatty‐liver‐disease
- 27. Liu J, Ayada I, Zhang X, et al. Estimating global prevalence of metabolic dysfunction‐associated fatty liver disease in overweight or obese adults. Clin Gastroenterol Hepatol 2022; 20: e573‐e582.
- 28. Ciardullo S, Monti T, Sala I, et al. Nonalcoholic fatty liver disease and advanced fibrosis in US adults across blood pressure categories. Hypertension 2020; 76: 562‐568.
- 29. Wang Z, Bertot LC, Jeffrey GP, et al. Serum fibrosis tests guide prognosis in metabolic dysfunction‐associated fatty liver disease patients referred from primary care. Clin Gastroenterol Hepatol 2022; 20: 2041‐2049.
- 30. Kim D, Konyn P, Sandhu KK, et al. Metabolic dysfunction‐associated fatty liver disease is associated with increased all‐cause mortality in the United States. J Hepatol 2021; 75: 1284‐1291.
- 31. Mantovani A, Csermely A, Tilg H, et al. Comparative effects of non‐alcoholic fatty liver disease and metabolic dysfunction‐associated fatty liver disease on risk of incident cardiovascular events: a meta‐analysis of about 13 million individuals. Gut 2023; 72: 1433‐1436.
- 32. Wijarnpreecha K, Lou S, Panjawatanan P, et al. Association between diastolic cardiac dysfunction and nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Dig Liver Dis 2018; 50: 1166‐1175.
- 33. Mantovani A, Pernigo M, Bergamini C, et al. Heart valve calcification in patients with type 2 diabetes and nonalcoholic fatty liver disease. Metabolism 2015; 64: 879‐887.
- 34. Targher G, Valbusa F, Bonapace S, et al. Non‐alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS One 2013; 8: e57183.
- 35. Commonwealth of Australia as represented by the Department of Health and Aged Care. Australian Guideline for assessing and managing cardiovascular disease risk. 2023. https://www.cvdcheck.org.au/overview (viewed Mar 2024).
- 36. Agustanti N, Soetedjo NNM, Damara FA, et al. The association between metabolic dysfunction‐associated fatty liver disease and chronic kidney disease: a systematic review and meta‐analysis. Diabetes Metab Syndr 2023; 17: 102780.
- 37. Ndumele CE, Neeland IJ, Tuttle KR, et al. A synopsis of the evidence for the science and clinical management of cardiovascular‐kidney‐metabolic (CKM) syndrome: a scientific statement from the American Heart Association. Circulation 2023; 148: 1636‐1664.
- 38. Mantovani A, Petracca G, Beatrice G, et al. Non‐alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta‐analysis. Gut 2022; 71: 156‐162.
- 39. Chen S, Pang J, Huang R, et al. Association of MAFLD with end‐stage kidney disease: a prospective study of 337,783 UK Biobank participants. Hepatol Int 2023; 17: 595‐605.
- 40. Chronic Kidney Disease (CKD) Management in Primary Care (5th edition). Kidney Health Australia, Melbourne, 2024. https://assets.kidney.org.au/resources/KHA‐CKD‐Handbook‐5th‐Ed‐July2024.pdf (viewed Mar 2024).
- 41. Quek J, Ng CH, Tang ASP, et al. Metabolic associated fatty liver disease increases the risk of systemic complications and mortality. a meta‐analysis and systematic review of 12 620 736 individuals. Endocr Pract 2022; 28: 667‐672.
- 42. Patel PJ, Hayward KL, Rudra R, et al. Multimorbidity and polypharmacy in diabetic patients with NAFLD: implications for disease severity and management. Medicine (Baltimore) 2017; 96: e6761.
- 43. Ng SSS, Wong VWS, Wong GLH, et al. Continuous positive airway pressure does not improve nonalcoholic fatty liver disease in patients with obstructive sleep apnea. a randomized clinical trial. Am J Respir Crit Care Med 2021; 203: 493‐501.
- 44. Hamilton GS, Chai‐Coetzer CL. Update on the assessment and investigation of adult obstructive sleep apnoea. Aust J Gen Pract 2019; 48: 176‐181.
- 45. Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology 2005; 129: 113‐121.
- 46. Thomas JA, Kendall BJ, El‐Serag HB, et al. Hepatocellular and extrahepatic cancer risk in people with non‐alcoholic fatty liver disease. Lancet Gastroenterol Hepatol 2024; 9: 159‐169.
- 47. Liebe R, Esposito I, Bock HH, et al. Diagnosis and management of secondary causes of steatohepatitis. J Hepatol 2021; 74: 1455‐1471.
- 48. Welfare AIoHa. Alcohol, tobacco and other drugs in Australia. 2023.
- 49. Seitz HK, Bataller R, Cortez‐Pinto H, et al. Alcoholic liver disease. Nat Rev Dis Primers 2018; 4: 16.
- 50. Magherman L, Van Parys R, Pauwels NS, et al. Meta‐analysis: the impact of light‐to‐moderate alcohol consumption on progressive non‐alcoholic fatty liver disease. Aliment Pharmacol Ther 2023; 57: 820‐836.
- 51. Naveau S, Giraud V, Borotto E, et al. Excess weight risk factor for alcoholic liver disease. Hepatology 1997; 25: 108‐111.
- 52. Raynard B, Balian A, Fallik D, et al. Risk factors of fibrosis in alcohol‐induced liver disease. Hepatology 2002; 35: 635‐638.
- 53. Marti‐Aguado D, Calleja JL, Vilar‐Gomez E, et al. Low‐to‐moderate alcohol consumption is associated with increased fibrosis in individuals with metabolic dysfunction‐associated steatotic liver disease. J Hepatol 2024; 81: 930‐940.
- 54. Australian Government National Health and Medical Research Council. Australian guidelines to reduce health risks from drinking alcohol. Canberra: Commonweatlh of Australia, NHMRC, 2020.
- 55. Vilar‐Gomez E, Calzadilla‐Bertot L, Wai‐Sun Wong V, et al. Fibrosis severity as a determinant of cause‐specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi‐national cohort study. Gastroenterology 2018; 155: 443‐457.
- 56. King J, McManus H, Kwon A, et al. HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2023: Sydney: The Kirby Institute, University of New South Wales, 2023.
- 57. King J, McManus H, Kwon A, et al. HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2022. Sydney: The Kirby Institute, University of New South Wales, 2022.
- 58. Koerbin G, Sikaris K, Jones GRD, et al. An update report on the harmonization of adult reference intervals in Australasia. Clin Chem Lab Med 2018; 57: 38‐41.
- 59. Buzzetti E, Petta S, Manuguerra R, et al. Evaluating the association of serum ferritin and hepatic iron with disease severity in non‐alcoholic fatty liver disease. Liver Int 2019; 39: 1325‐1334.
- 60. Bugianesi E, Manzini P, D’Antico S, et al. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology 2004; 39: 179‐187.
- 61. Adams LA, Crawford DH, Stuart K, et al. The impact of phlebotomy in nonalcoholic fatty liver disease: a prospective, randomized, controlled trial. Hepatology 2015; 61: 1555‐1564.
- 62. Laine F, Ruivard M, Loustaud‐Ratti V, et al. Metabolic and hepatic effects of bloodletting in dysmetabolic iron overload syndrome: a randomized controlled study in 274 patients. Hepatology 2017; 65: 465‐474.
- 63. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313‐1321.
- 64. Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int 2021; 41: 1290‐1293.
- 65. Vaz K, Kemp W, Majeed A, et al. Non‐alcoholic fatty liver disease prevalence in Australia has risen over 15‐years in conjunction with increased prevalence of obesity and reduction in healthy lifestyle. J Gastroenterol Hepatol 2023; 38: 1823‐1831.
- 66. Patel P, Hossain F, Horsfall LU, et al. A pragmatic approach identifies a high rate of nonalcoholic fatty liver disease with advanced fibrosis in diabetes clinics and at‐risk populations in primary care. Hepatol Commun 2018; 2: 893‐905.
- 67. Gawrieh S, Wilson LA, Cummings OW, et al. Histologic findings of advanced fibrosis and cirrhosis in patients with nonalcoholic fatty liver disease who have normal aminotransferase levels. Am J Gastroenterol 2019; 114: 1626‐1635.
- 68. Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003; 37: 1286‐1292.
- 69. Hetland LE, Kronborg TM, Thing M, et al. Suboptimal diagnostic accuracy of ultrasound and CT for compensated cirrhosis: evidence from prospective cohort studies. Hepatol Commun 2023; 7: e0231.
- 70. Mozes FE, Lee JA, Selvaraj EA, et al. Diagnostic accuracy of non‐invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta‐analysis. Gut 2022; 71: 1006‐1019.
- 71. McPherson S, Hardy T, Dufour JF, et al. Age as a confounding factor for the accurate non‐invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017; 112: 740‐751.
- 72. Hayward KL, McKillen BJ, Horsfall LU, et al. Towards collaborative management of non‐alcoholic fatty liver disease: a ‘real‐world’ pathway for fibrosis risk assessment in primary care. Intern Med J 2022; 52: 1749‐1758.
- 73. Boursier J, Hagstrom H, Ekstedt M, et al. Non‐invasive tests accurately stratify patients with NAFLD based on their risk of liver‐related events. J Hepatol 2022; 76: 1013‐1020.
- 74. Selvaraj EA, Mozes FE, Jayaswal ANA, et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: a systematic review and meta‐analysis. J Hepatol 2021; 75: 770‐785.
- 75. Cassinotto C, Boursier J, de Ledinghen V, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology 2016; 63: 1817‐1827.
- 76. Cassinotto C, Boursier J, Paisant A, et al. Transient versus two‐dimensional shear‐wave elastography in a multistep strategy to detect advanced fibrosis in NAFLD. Hepatology 2021; 73: 2196‐2205.
- 77. Kemp W, Roberts S. FibroScan(R) and transient elastography. Aust Fam Physician 2013; 42: 468‐471.
- 78. Kemp W, Roberts S. Feasibility and performance of the FibroScan XL probe. Hepatology 2012; 55: 1308‐1309; author reply 1309‐1310.
- 79. Boursier J, Cassinotto C, Hunault G, et al. Criteria to determine reliability of noninvasive assessment of liver fibrosis with virtual touch quantification. Clin Gastroenterol Hepatol 2019; 17: 164‐171.
- 80. Adams LA, George J, Bugianesi E, et al. Complex non‐invasive fibrosis models are more accurate than simple models in non‐alcoholic fatty liver disease. J Gastroenterol Hepatol 2011; 26: 1536‐1543.
- 81. Vali Y, Lee J, Boursier J, et al. Biomarkers for staging fibrosis and non‐alcoholic steatohepatitis in non‐alcoholic fatty liver disease (the LITMUS project): a comparative diagnostic accuracy study. Lancet Gastroenterol Hepatol 2023; 8: 714‐725.
- 82. Vali Y, Lee J, Boursier J, et al. Enhanced liver fibrosis test for the non‐invasive diagnosis of fibrosis in patients with NAFLD: a systematic review and meta‐analysis. J Hepatol 2020; 73: 252‐262.
- 83. Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non‐alcoholic fatty liver disease. J Hepatol 2019; 71: 371‐378.
- 84. Huang Y, Joseph J, de Boer WB, et al. Long‐term liver‐related outcomes of patients with chronic liver diseases in Australia. Clin Gastroenterol Hepatol 2020; 18: 496‐504.
- 85. Bertot LC, Jeffrey GP, de Boer B, et al. Comparative accuracy of clinical fibrosis markers, Hepascore and Fibroscan(R) to detect advanced fibrosis in patients with nonalcoholic fatty liver disease. Dig Dis Sci 2023; 68: 2757‐2767.
- 86. Boursier J, Guillaume M, Leroy V, et al. New sequential combinations of non‐invasive fibrosis tests provide an accurate diagnosis of advanced fibrosis in NAFLD. J Hepatol 2019; 71: 389‐396.
- 87. Bertot LC, Jeffrey GP, Wallace M, et al. Nonalcoholic fatty liver disease‐related cirrhosis is commonly unrecognized and associated with hepatocellular carcinoma. Hepatol Commun 2017; 1: 53‐60.
- 88. Simon TG, Roelstraete B, Hagstrom H, et al. Progression of non‐alcoholic fatty liver disease and long‐term outcomes: a nationwide paired liver biopsy cohort study. J Hepatol 2023; 79: 1366‐1373.
- 89. Cholankeril G, Kramer JR, Chu J, et al. Longitudinal changes in fibrosis markers are associated with risk of cirrhosis and hepatocellular carcinoma in non‐alcoholic fatty liver disease. J Hepatol 2023; 78: 493‐500.
- 90. Hagstrom H, Talback M, Andreasson A, et al. Repeated FIB‐4 measurements can help identify individuals at risk of severe liver disease. J Hepatol 2020; 73: 1023‐1029.
- 91. Kleiner DE, Brunt EM, Wilson LA, et al. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open 2019; 2: e1912565.
- 92. Alexopoulos AS, Crowley MJ, Wang Y, et al. Glycemic control predicts severity of hepatocyte ballooning and hepatic fibrosis in nonalcoholic fatty liver disease. Hepatology 2021; 74: 1220‐1233.
- 93. Huang DQ, Wilson LA, Behling C, et al. Fibrosis progression rate in biopsy‐proven nonalcoholic fatty liver disease among people with diabetes versus people without diabetes: a multicenter study. Gastroenterology 2023; 165: 463‐472.
- 94. van Kleef LA, Sonneveld MJ, Kavousi M, et al. Fatty liver disease is not associated with increased mortality in the elderly: a prospective cohort study. Hepatology 2023; 77: 585‐593.
- 95. Golabi P, Paik J, Reddy R, et al. Prevalence and long‐term outcomes of non‐alcoholic fatty liver disease among elderly individuals from the United States. BMC Gastroenterol 2019; 19: 56.
- 96. Hoshida Y, Ikeda K, Kobayashi M, et al. Chronic liver disease in the extremely elderly of 80 years or more: clinical characteristics, prognosis and patient survival analysis. J Hepatol 1999; 31: 860‐866.
- 97. Orci LA, Sanduzzi‐Zamparelli M, Caballol B, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta‐analysis, and meta‐regression. Clin Gastroenterol Hepatol 2022; 20: 283‐292.
- 98. Cancer Council Australia Hepatocellular Carcinoma Surveillance Working Group. Clinical practice guidelines for hepatocellular carcinoma surveillance for people at high risk in Australia. Sydney: Cancer Council Australia, 2023.
- 99. Singal AG, El‐Serag HB. Rational HCC screening approaches for patients with NAFLD. J Hepatol 2022; 76: 195‐201.
- 100. Hong TP, Gow PJ, Fink M, et al. Surveillance improves survival of patients with hepatocellular carcinoma: a prospective population‐based study. Med J Aust 2018; 209: 348‐354. https://www.mja.com.au/journal/2018/209/8/surveillance‐improves‐survival‐patients‐hepatocellular‐carcinoma‐prospective
- 101. Huang Y, Wallace MC, Adams LA, et al. Rate of nonsurveillance and advanced hepatocellular carcinoma at diagnosis in chronic liver disease. J Clin Gastroenterol 2018; 52: 551‐556.
- 102. Taddei TH, Jou JH. When to stop screening‐liver cancer. Am J Gastroenterol 2023; 118: 427‐428.
- 103. Koutoukidis DA, Jebb SA, Tomlinson JW, et al. Association of weight changes with changes in histological features and blood markers in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2022; 20: e538‐e547.
- 104. Aminian A, Al‐Kurd A, Wilson R, et al. Association of bariatric surgery with major adverse liver and cardiovascular outcomes in patients with biopsy‐proven nonalcoholic steatohepatitis. JAMA 2021; 326: 2031‐2042.
- 105. Mantovani A, Petracca G, Beatrice G, et al. Non‐alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta‐analysis of 501022 adult individuals. Gut 2021; 70: 962‐969.
- 106. Gao Y, Zhao T, Song S, et al. Lean nonalcoholic fatty liver disease and risk of incident type 2 diabetes mellitus: a literature review and meta‐analysis. Diabetes Res Clin Pract 2023; 200: 110699.
- 107. Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Rep 2019; 1: 312‐328.
- 108. Arase Y, Suzuki F, Ikeda K, et al. Multivariate analysis of risk factors for the development of type 2 diabetes in nonalcoholic fatty liver disease. J Gastroenterol 2009; 44: 1064‐1070.
- 109. Cao L, An Y, Liu H, et al. Global epidemiology of type 2 diabetes in patients with NAFLD or MAFLD: a systematic review and meta‐analysis. BMC Med 2024; 22: 101.






Abstract
Introduction: Metabolic dysfunction‐associated fatty liver disease (MAFLD) is common. This evidence‐based consensus statement summary provides recommendations for the assessment and monitoring of adults with MAFLD in primary care.
Main recommendations: Adults with type 2 diabetes, obesity or two or more other metabolic risk factors should be tested for MAFLD. Hepatic steatosis should be evaluated using ultrasound, whereas the presence and complications of type 2 diabetes and obesity should be assessed according to current Australian guidelines. Cardiovascular disease, chronic kidney disease and obstructive sleep apnoea are common in people with MAFLD and should be considered as part of a holistic health assessment. Alternative causes of hepatic steatosis, including excess alcohol consumption, must be considered, and patients with elevated serum aminotransferase levels should be tested for hepatitis B and C infection and iron overload. The risk of advanced liver fibrosis requires assessment using the Fibrosis‐4 (FIB‐4) Index; a low score (< 1.3) is associated with a more than 95% negative predictive value for advanced liver fibrosis. People with an indeterminate FIB‐4 score (between 1.3 and 2.7) should undergo second‐line assessment with liver elastography or a direct liver fibrosis serum test or, if these tests are unavailable, should be referred to an expert clinician in liver disease. People with MAFLD and a high FIB‐4 score (> 2.7), an elevated direct liver fibrosis serum test, high elastography results or with clinical, laboratory or imaging evidence of cirrhosis should be referred for further evaluation. Individuals with a low FIB‐4 score (< 1.3), low elastography or direct liver fibrosis serum test results should be monitored with a repeat FIB‐4 test at least every three years. Monitoring of weight, body mass index and/or waist circumference and for emergence of type 2 diabetes (in individuals without) should be performed at least annually.
Change in management as a result of this consensus statement summary: Appropriate identification, assessment and risk stratification of people with MAFLD will aid referral pathways, further investigation and management.