The incidence and impact of primary liver cancer in Australia is growing. Over the past two decades, there have been major gains in the fight against cancer in Australia. Despite our ageing population, overall cancer mortality has decreased.1 Yet, primary liver cancer remains an outlier to this trend: in 2019, the estimated incidence of primary liver cancer was more than four times higher (10.1 cases per 100 000) than in 1982 (2.2 cases per 100 000), a faster increase than any other type of cancer in Australia.1 Primary liver cancer is also a low survival cancer, with a five‐year survival of only 22%.1
Hepatocellular carcinoma (HCC) accounts for over 85% of primary liver cancer,2,3 and usually develops in the setting of chronic liver disease, with up to 90% of cases occurring in people with liver cirrhosis.3 Previously, viral hepatitis and alcohol‐related liver disease were the most common aetiologies of HCC.4 However, metabolic dysfunction‐associated fatty liver disease (MAFLD) is rapidly emerging as a major cause of HCC.5 In contrast to many other cancers, the at‐risk population for HCC is well defined.6 Moreover, when detected at an early stage, patients can be offered curative treatments.3 Surveillance for HCC in individuals at risk with six‐monthly liver ultrasound scans, with or without the α‐fetoprotein (AFP) blood test, improves HCC survival, is recommended in guidelines and is cost‐effective in the Australian context.7,8
New strategies are urgently needed to reduce deaths from liver cancer in Australia
Due to a low uptake of surveillance, most HCC cases continue to be detected at an advanced stage when only palliative treatments can be offered. Data from Melbourne show minimal change in the proportion of new HCC cases diagnosed through surveillance over the past decade, from 40% in 2013 to 39% in 2022.9,10 This is primarily due to lack of awareness among health workers and the community of who is at risk of chronic liver disease and HCC, the asymptomatic nature of chronic liver disease and HCC in the early stages, lack of awareness of the survival benefits of HCC surveillance for individuals at risk, and lack of access to HCC surveillance.11 The impact of late diagnosis of HCC on mortality is even more striking among people who face barriers to care, including First Nations Australians and those who experience social and health disadvantages.12
Yet, in some east Asian countries, between 2000 and 2020, both the incidence and mortality of primary liver cancer have fallen, reflecting successful preventive health initiatives that address viral hepatitis, such as hepatitis B vaccination and treatment, and hepatitis C cure.2,13 However, improvements in HCC mortality may also be driven by early diagnosis and increased use of curative treatment.14 Taking Japan as an example, access to HCC surveillance and improvements in diagnostic methods and treatments have seen a dramatic improvement in five‐year survival from 5% in 1980 up to 43% in 2005 and 58% in 2013 (Box 1). In Australia, implementation of advances in public health interventions for chronic liver disease, surveillance, diagnostic methods and treatments has followed a similar trajectory to Japan, but HCC survival has remained stubbornly low (22%, Box 2). What can Australia learn from countries such as Japan, who have seen such impressive reductions in HCC mortality?
Hepatocellular carcinoma prevention and surveillance strategies: comparing Japan to Australia
National hepatocellular carcinoma registry and surveillance program
There are several critical differences in the Japanese approach to HCC compared with Australia that likely contribute to lower HCC mortality (Box 3). The first and arguably most important is that since the 1980s, Japan has had a national HCC surveillance program.13 Annual education of general practitioners and health promotion campaigns for the general population about HCC and its risk factors have been supported and financed by the Japanese Ministry of Health since 1999.13 Importantly, this program was embedded within pre‐existing national screening and treatment programs for viral hepatitis, facilitating early assessment for liver cirrhosis and linkage into HCC surveillance.13 In Australia, we have national surveillance programs for bowel, cervical and breast cancer;15 however we have no national population‐level screening programs for chronic liver disease and no national HCC surveillance program.
Population‐level screening for chronic liver disease alone is not enough to increase HCC surveillance uptake; dedicated resources and health policy change to support HCC surveillance are required. New Zealand has a national screening and monitoring program for people living with chronic hepatitis B; however, relatively few patients undergo HCC surveillance through this program (26%).16 In an Australian study of HCC surveillance in people known to have chronic hepatitis B in primary care, only 27% had optimal adherence.17 Global estimates are similar, with patients attending specialist hepatology services having significantly higher adherence to HCC surveillance regimens, due in part to challenges in delivery of timely HCC surveillance in primary care, such as lack of awareness, out‐of‐pocket costs and opportunistic use of primary care services by patients.18
Cornerstones of Japan's successful HCC program include delivery of surveillance via a national HCC risk registry and community‐based HCC surveillance centres and hospital settings at a minimal cost.13 Although Japan has fewer geographical challenges to negotiate in delivery of its HCC program compared with Australia, centralised organisation of HCC surveillance with decentralised imaging delivery in clinics has proven effective to improve access to high quality HCC surveillance.13 Data from Victoria showed that during the coronavirus disease 2019 pandemic, there was a shift in use of hospital to community‐based radiology services to deliver hepatology clinic‐led HCC surveillance programs, without a significant reduction in HCC detection rates, suggesting a similar hub and spoke model could work well nationwide.19
Use of blood biomarkers to enhance hepatocellular carcinoma surveillance accuracy and uptake
The second major difference in the approach to HCC surveillance in Japan is the use of additional blood biomarkers to boost the sensitivity of ultrasound scans for HCC detection that are not available in Australia. These blood biomarkers include Lens culinaris agglutinin‐reactive fraction of fetoprotein (AFP‐L3), which has been available in Japan since 1996, and protein induced by vitamin K absence‐II (PIVKA‐II) since 1998.13,20 These blood biomarkers, alongside a second blood test, are used to enhance early recurrence detection after treatment.21 Serum AFP, used widely in HCC surveillance, can be elevated in non‐tumorous hepatic disorders or may reflect general hepatic inflammatory and regenerative activity.21 PIVKA‐II helps differentiate HCC from other hepatic diseases, and is independent of AFP.21 A systematic review of 49 studies noted a higher performance of PIVKA‐II and AFP combined for HCC detection (area under the receiver operating curve, 0.88) compared with PIVKA‐II (0.83) and AFP (0.77) alone.22 These two biomarkers were combined into the GALAD score (gender, age, AFP‐L3, AFP and PIVKA‐II), which has excellent accuracy for early‐stage HCC detection in combination with an abdominal ultrasound scan (Box 4).23 In 2018, a multicentre study showed superior performance of GALAD alone compared with liver ultrasound for HCC detection (GALAD alone sensitivity 91% and specificity 85%), with further improved performance when the modalities were combined (GALADUS [GALAD and ultrasound] sensitivity 95%, specificity 91%).24 Recently, a new GAAD score without AFP‐L3 (gender, age, AFP and PIVKA‐II) has shown comparable performance to GALAD in an international cohort study for detection of HCC in people with chronic liver disease (73% sensitivity GAAD versus 74% GALAD for all‐stage HCC).25 Emerging evidence suggests use of GAAD in place of AFP, with or without ultrasound, is cost‐effective in some settings.26,27
Unlike Japan where there is a relatively low incidence of obesity compared with other high resource countries,28 in Australia increasing incidence and prevalence of MAFLD is expected to present significant challenges to HCC surveillance. MAFLD is strongly associated with obesity, which can lead to reduced sensitivity of liver ultrasound for HCC detection.29 HCC can also occur in people with MAFLD and hepatitis B in the absence of cirrhosis.30 Access to blood biomarkers could help stratify HCC risk and improve surveillance effectiveness among people with MAFLD and obesity, which is an urgent issue based on projections suggesting a considerable proportion of Australians will develop MAFLD in the future and the lack of biomarkers for HCC prediction in those with non‐cirrhotic MAFLD.30,31
Blood biomarker HCC surveillance strategies could also revolutionise HCC surveillance coverage in remote and very remote parts of Australia, where access to radiology services is limited. Using a blood‐based biomarker approach for surveillance to triage the need for further imaging would be a game changer to expand access to timely HCC diagnosis in remote Australia.
In addition to GAAD for HCC surveillance, other novel biofluid‐based biomarkers such as circulating tumour cells and urine metabolomics may prove effective for early HCC detection and prognosis in the future, but are not currently ready for clinical use.
Tiered, risk‐based approach to HCC surveillance in Japan
Although the target populations for surveillance in Japan are similar to Australian criteria,32 the Japanese HCC surveillance uses liver ultrasound coupled with additional blood biomarkers at risk‐based intervals (Box 3).32 Those with viral hepatitis‐related cirrhosis are considered very high risk for HCC and have short‐interval surveillance every three to four months, combined with a dynamic computed tomography (CT) or magnetic resonance imaging (MRI) scan supported at clinician discretion.32 This contrasts with Australia and other countries, where six‐monthly ultrasound scans with or without AFP are used for people at risk of HCC.7 Currently, evidence is lacking to support efficacy or cost‐effectiveness of short interval ultrasound and alternative imaging with CT and MRI for HCC. However, new strategies such as short interval use of blood biomarkers with six‐monthly ultrasound scans merit exploration in high quality studies. Personalised approaches to HCC surveillance may prove more effective in some settings. Data show that short‐sequence MRI without contrast outperforms ultrasound accuracy for HCC detection in high risk patients;33 however, in Australia access to MRI is limited and this is unlikely to be a feasible approach. In addition, there may be a role for risk calculators to stratify risk in patients with hepatitis B, such as REAL‐B, PAGE‐B and REACH‐B.
Actions to improve primary liver cancer survival in Australia: lessons from Japan
Based on what has proven effective in Japan, Box 5 presents a list of five key actions that Australia needs to take to reduce deaths from primary liver cancer.
Support a nationwide campaign for liver disease health promotion in partnership with community organisations
In Japan, nationwide government‐funded liver disease and liver cancer health promotion campaigns have been essential to the success of their HCC surveillance program. In Australia, the diversity of chronic liver disease aetiologies and affected communities necessitate community‐led, co‐designed health promotion campaigns to address stigma and reduce discrimination, particularly among culturally and linguistically diverse communities and First Nations Australians.
Establish and fund a national approach to chronic liver disease screening and linkage to care, including timely access to liver fibrosis assessment
In Japan, the national viral hepatitis screening programs have proven effective in identifying those at risk of HCC. In Australia, regardless of the cause of liver disease, population screening approaches through primary care are likely to be effective and help destigmatise chronic liver disease. However, testing for chronic liver disease is only one facet of reducing HCC risk; for example, in Australians with hepatitis B: while 72% are estimated to be diagnosed, only 26% are linked to care,34 highlighting that without effective means of linking and retaining people with chronic liver disease in care, testing alone will not increase HCC surveillance uptake.
Assessment of risks for chronic liver disease, patient education and testing require time and training. Creating a specific funding item for liver disease assessment would support general practitioners and nurse practitioners to provide comprehensive liver assessments in primary care and provide impetus for these assessments to be incorporated into clinic key performance indicators, as done for chronic kidney disease and type 2 diabetes risk assessments. Given the complexity and diversity of disease screening requirements for general practitioners in a rapidly changing practice landscape, electronic decision support tools embedded into electronic medical record systems can support general practitioners with risk profiling, testing and management algorithms, including automation of referral pathways.
Evaluation of the degree of liver scarring (fibrosis) is an essential component of liver disease assessment and management. We recommend initial screening for cirrhosis using algorithms calculated from routine blood tests, such as aspartate transaminase to platelet ratio index (APRI) or fibrosis‐4 (FIB‐4), followed by FibroScan (Echosens) or other non‐invasive assessment of liver fibrosis. Automated reporting of APRI or FIB‐4 within general practitioner electronic medical records on routine blood tests is one way to increase cirrhosis screening in primary care. Other blood‐based biomarkers for assessment of cirrhosis are available in some states (eg, Western Australia, Queensland) but are not reimbursed by Medicare.
In contrast to Japan, access to transient elastography and other non‐invasive means of diagnosing cirrhosis is unequally distributed and unfunded nationally, with no Medicare Benefits Schedule (MBS) item for fibrosis assessment, despite it being the critical step in identifying individuals needing HCC surveillance. National funding of transient elastography through MBS would facilitate cirrhosis diagnosis, addressing inequities in linkage to HCC surveillance across Australia, including for First Nations Australians in remote areas.
Establish and fund a national HCC surveillance program and HCC registry
A centralised operational approach coupled with community access points is vital for a country like Australia with the geographical vastness and relatively small population. An HCC surveillance registry is feasible due to the population at risk being only a fraction of the whole population, and the surveillance tests required being non‐invasive and relatively low cost, compared with other national cancer surveillance programs such as for colorectal cancer and the newly established lung cancer screening programs. Evidence shows that targeted liver ultrasound is more effective and accurate for HCC diagnosis than general abdominal ultrasound35 and having a liver ultrasound performed by experienced technicians at the same centre over time also improves surveillance accuracy, particularly for early‐stage tumours.36 Having accredited radiology services with increased training providing HCC surveillance through a national program may aid in ensuring high quality surveillance. While HCC surveillance may increase patient anxiety and harms through further testing, it is generally very safe and acceptable to people at risk.14
Fast‐track approval and integration of blood biomarkers into HCC surveillance programs
There is emerging evidence supporting the accuracy of GAAD in addition to liver ultrasound to improve early HCC diagnosis internationally (Box 4) and in Australia.14,37,38,39 Rapid assessment, approval and MBS subsidy of blood biomarkers for primary liver cancer surveillance, diagnosis and prognosis will facilitate the patient's rapid linkage into clinical care, particularly in remote areas.
Set clear targets for liver disease screening and assessment, HCC surveillance uptake and HCC mortality
Setting national targets in disease management helps galvanise policy action and investment towards achieving these goals, as demonstrated by the World Health Organization 2030 viral hepatitis global targets set for testing, linkage to care, treatment and mortality, which informed the hepatitis B and C strategy targets in Australia.40 Such aspirational goals should be set for the cascade of care in primary liver cancer as part of the Australian Roadmap to Liver Cancer Control to improve survival in HCC.11
Conclusion
Despite access to advanced treatments for HCC in Australia, the potential for curative outcomes is limited by how early cancer is detected. Adopting health policy change that increases assessment for liver disease and cirrhosis in primary care, coupled with a strong, funded national HCC surveillance program using ultrasound and blood biomarkers is vital to reduce deaths from HCC.
Box 1 – Changes in liver cancer five‐year survival rate, adapted from Kudo 202311
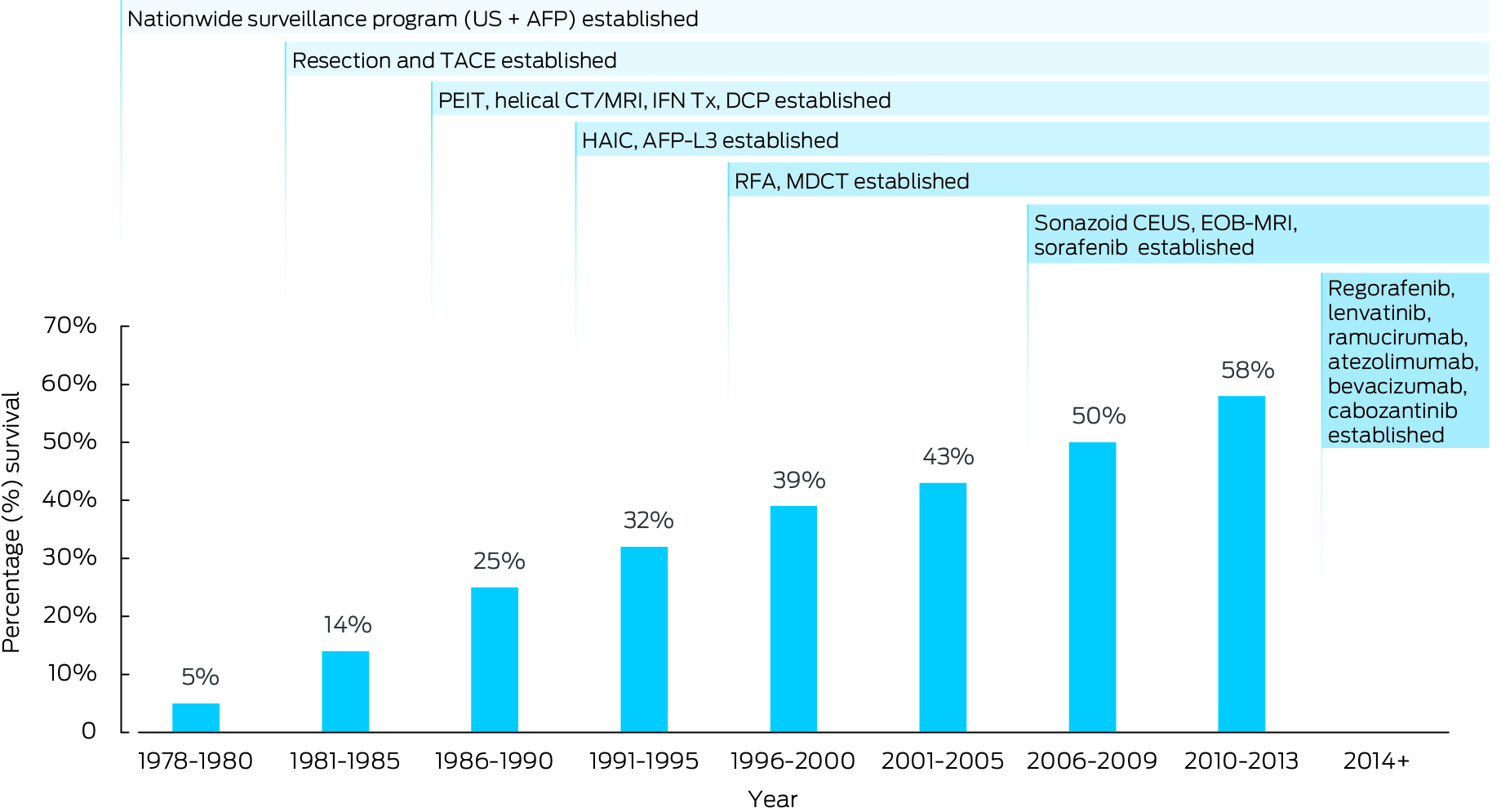
AFP = α‐fetoprotein; AFP‐L3, Lens culinaris agglutinin‐reactive fraction of fetoprotein; CEUS = contrast‐enhanced ultrasound; CT = computed tomography; DCP = des‐γ‐carboxy prothrombin; EOB‐MRI = gadolinium‐ethoxybenzyl‐diethylenetriamine pentaacetic acid magnetic resonance imaging; HAIC = hepatic arterial infusion chemotherapy; IFN Tx = interferon treatment; MDCT = multi‐detector computed tomography; MRI = magnetic resonance imaging; PEIT = percutaneous ethanol injection technique; RFA = radiofrequency ablation; TACE = transcatheter arterial chemoembolisation; US = ultrasound. This picture has been modified from the original from Masatoshi Kudo; Surveillance, diagnosis, and treatment outcome of hepatocellular carcinoma in Japan: 2023 Update. Liver Cancer 2023; 12: 95‐102. https://doi.org/10.1159/000530079.
Box 2 – Changes in liver cancer five‐year survival rate in Australia13,14,36
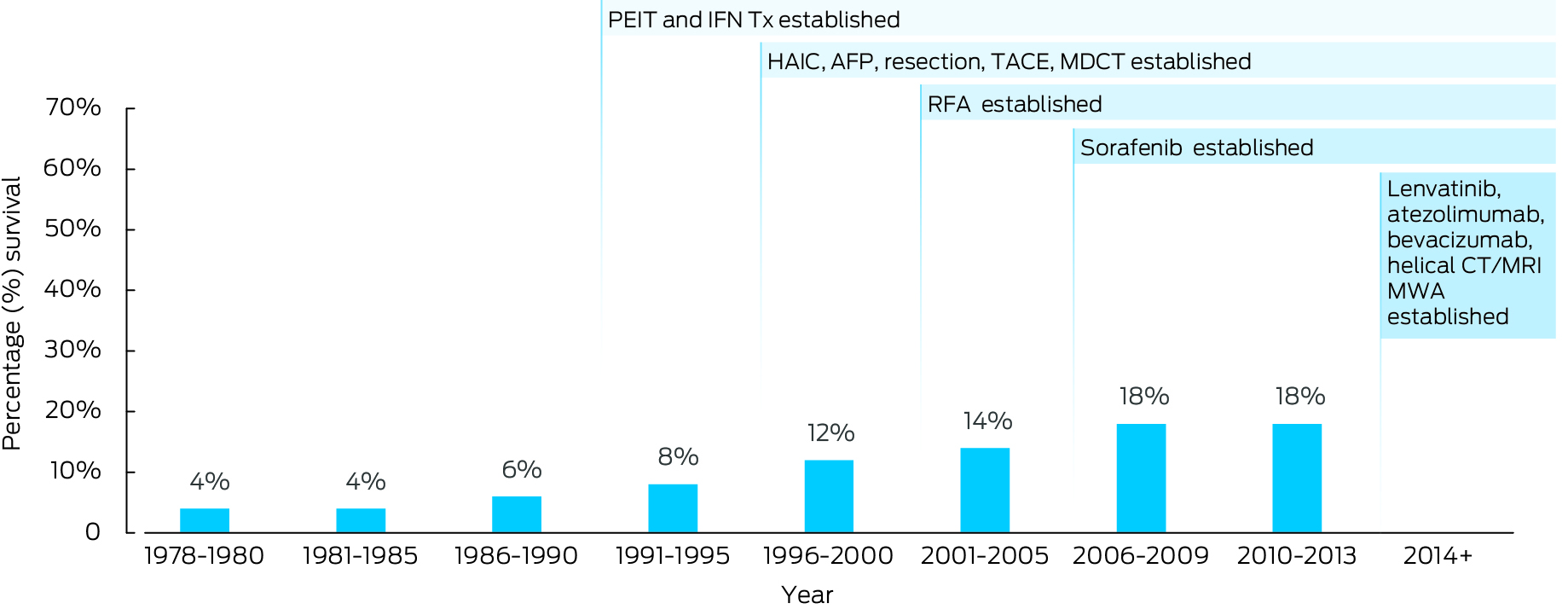
AFP = α‐fetoprotein; AFP‐L3, Lens culinaris agglutinin‐reactive fraction of fetoprotein; CT = computed tomography; HAIC = hepatic arterial infusion chemotherapy; IFN Tx = interferon treatment; MDCT = multi‐detector computed tomography; MRI = magnetic resonance imaging; MWA = microwave ablation; PEIT = percutaneous ethanol injection technique; RFA = radiofrequency ablation; TACE = transcatheter arterial chemoembolisation.
Box 3 – Hepatocellular carcinoma surveillance approaches, comparing Japan and Australia
|
Japan |
Australia |
||||||||||||||
|
|
|||||||||||||||
|
National surveillance program |
Clinician‐initiated surveillance |
||||||||||||||
|
Free hepatitis B and C testing, automated linkage to care hospitals |
Free hepatitis B and C testing, some automated linkage programs |
||||||||||||||
|
Hospitals, general practitioners, small community health centres can access national program |
Health workers arrange themselves |
||||||||||||||
|
PIVKA‐II, AFP‐L3 and AFP fully funded in national surveillance program |
AFP available and funded |
||||||||||||||
|
Ultrasound funded |
Ultrasound reimbursed (Medicare) |
||||||||||||||
|
Hepatologist performs liver ultrasound |
Radiologist/radiographer performs liver ultrasound |
||||||||||||||
Different surveillance strategies:
|
Six‐monthly ultrasound ± AFP for at‐risk patients |
||||||||||||||
|
|
|||||||||||||||
|
AFP = α‐fetoprotein; AFP‐L3 = Lens culinaris agglutinin‐reactive fraction of fetoprotein; CT = computed tomography; HBV = hepatitis B virus; MRI = magnetic resonance imaging; PIVKA‐II = protein induced by vitamin K absence‐II. |
|||||||||||||||
Box 4 – Summary of blood‐based biomarkers in hepatocellular carcinoma surveillance
|
|
|||||||||||||||
|
Ultrasound* |
|
|
|
||||||||||||
|
Group |
Pooled sensitivity (95% CI) |
Pooled specificity (95% CI) |
AUC (95% CI) |
||||||||||||
|
Any‐stage HCC within Milan criteria |
0.84 (0.76–0.92) |
0.94 (0.90–0.97) |
0.96 (0.94–0.98) |
||||||||||||
|
Early‐stage HCC within Milan criteria |
0.47 (0.33–0.61) |
0.91 (0.86–0.94) |
0.88 (0.85–0.90) |
||||||||||||
|
GALAD † |
|
|
|
||||||||||||
|
|
Pooled sensitivity (95% CI) |
Pooled specificity (95% CI) |
AUC (95% CI) |
||||||||||||
|
Any‐stage HCC |
0.82 (0.78–0.85) |
0.89 (0.85–0.91) |
0.92 (0.89–0.94) |
||||||||||||
|
Within BCLC 0/A |
0.73 (0.66–0.79) |
0.87 (0.81–0.91) |
0.86 (0.82–0.88) |
||||||||||||
|
Within Milan criteria |
0.65 (0.56–0.72) |
0.91 (0.87–0.94) |
0.87 (0.83–0.89) |
||||||||||||
|
HBV |
0.76 (0.69–0.81) |
0.96 (0.87–0.99) |
0.85 (0.82–0.88) |
||||||||||||
|
HCV |
0.86 (0.80–0.91) |
0.89 (0.83–0.93) |
0.94 (0.91–0.95) |
||||||||||||
|
Non‐viral liver diseases |
0.87 (0.81–0.91) |
0.91 (0.85–0.95) |
0.94 (0.92–0.96) |
||||||||||||
|
Cirrhosis |
0.76 (0.69–0.82) |
0.85 (0.79–0.90) |
0.87 (0.83–0.89) |
||||||||||||
|
Western countries |
0.85 (0.79–0.89) |
0.88 (0.84–0.91) |
0.93 (0.91–0.95) |
||||||||||||
|
East Asian countries |
0.76 (0.70–0.81) |
0.91 (0.79–0.96) |
0.85 (0.81–0.87) |
||||||||||||
|
Countries from different continents |
0.79 (0.73–0.84) |
0.89 (0.86–0.91) |
0.91 (0.89–0.94) |
||||||||||||
|
GAAD ‡ |
|
|
|
||||||||||||
|
|
Sensitivity (95% CI) |
Specificity (95% CI) |
AUC (95% CI) |
||||||||||||
|
Any‐stage HCC |
0.85 (0.81–0.89) |
0.90 (0.88–0.92) |
0.95 (0.94–0.96) |
||||||||||||
|
Early‐stage HCC |
0.72 (0.63–0.80) |
|
0.91 (0.88–0.93) |
||||||||||||
|
|
|||||||||||||||
|
AFP = α‐fetoprotein; AFP‐L3 = Lens culinaris agglutinin‐reactive fraction of fetoprotein; AUC = area under the curve; BCLC = Barcelona clinic liver cancer; CI = confidence interval; GAAD = gender, age, AFP and PIVKA‐II; GALAD = combining gender and age plus a three‐serum biomarker panel of AFP‐L3, AFP and PIVKA‐II; HBV = hepatitis B virus; HCC = hepatocellular carcinoma; HCV = hepatitis C virus; PIVKA‐II = protein induced by vitamin K absence‐II. * Adapted from Tzartzeva et al,39 across 32 studies in 13 367 patients. † Adapted from Guan et al,23 across 15 studies with 19 021 patients. ‡ Adapted from Piratvisuth et al,37 validation studies in 1084 patients with 309 cases HCC. |
|||||||||||||||
Box 5 – Five key actions to reduce deaths from primary liver cancer
|
|
Action |
||||||||||||||
|
|
|||||||||||||||
|
1 |
Support a nationwide campaign for liver disease health promotion in partnership with community organisations |
||||||||||||||
|
2 |
Establish and fund a national approach to chronic liver disease screening and linkage to care, including timely access to liver fibrosis assessment |
||||||||||||||
|
3 |
Establish and fund a national HCC risk registry and surveillance program |
||||||||||||||
|
4 |
Fast‐track approval and integration of blood‐based biomarkers into HCC surveillance programs |
||||||||||||||
|
5 |
Set clear targets for liver disease screening and assessment, HCC surveillance uptake and HCC mortality |
||||||||||||||
|
|
|||||||||||||||
|
HCC = hepatocellular carcinoma. |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- 1. Australian Government Australian Institute of Health and Welfare. Cancer data in Australia [website]. Canberra: AIHW, 2023. https://www.aihw.gov.au/reports/cancer/cancer‐data‐in‐australia/contents/overview‐of‐cancer‐in‐australia‐2023 (viewed Dec 2023).
- 2. Arnold M, Abnet CC, Neale RE, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020; 159: 335‐349.
- 3. Lubel JS, Roberts SK, Strasser SI, et al. Australian recommendations for the management of hepatocellular carcinoma: a consensus statement. Med J Aust 2021; 214: 475‐483. https://www.mja.com.au/journal/2021/214/10/australian‐recommendations‐management‐hepatocellular‐carcinoma‐consensus
- 4. Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol 2017; 3: 1683‐1691.
- 5. Gastroenterological Society of Australia. Recommendations for the assessment of metabolic dysfunction‐associated fatty liver disease (MAFLD) in primary care [website]. https://www.gesa.org.au/public/13/files/Education%20&%20Resources/Clinical%20Practice%20Resources/MAFLD/MAFLD%20consensus%20statement%202024.pdf (viewed Aug 2024).
- 6. Tang A, Hallouch O, Chernyak V, et al. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY) 2018; 43: 13‐25.
- 7. Cancer Council Australia Hepatocellular Carcinoma Surveillance Working Group. Clinical practice guidelines for hepatocellular carcinoma surveillance for people at high risk in Australia [website]. https://www.cancer.org.au/clinical‐guidelines/liver‐cancer/hepatocellular‐carcinoma (viewed May 2024).
- 8. Nguyen ALT, Si L, Lubel JS, et al. Hepatocellular carcinoma surveillance based on the Australian Consensus Guidelines: a health economic modelling study. BMC Health Serv Res 2023; 23: 378.
- 9. Flores JE, Thompson AJV, Lo SW, et al. Changing epidemiology of HCC in Melbourne, Australia: primary outcomes of the prospective HOMER‐2 cohort. Hepatology 2023; 78: S1809‐S1810.
- 10. Hong TP, Gow P, Fink M, et al. Novel population‐based study finding higher than reported hepatocellular carcinoma incidence suggests an updated approach is needed. Hepatology 2016; 63: 1205‐1212.
- 11. Cancer Council Australia. Roadmap to liver cancer control in Australia. Cancer Council Australia, 2023. https://www.cancer.org.au/assets/pdf/roadmap‐to‐liver‐cancer‐control‐in‐australia (viewed Aug 2024).
- 12. Howell J, Combo T, Binks P, et al. Overcoming disparities in hepatocellular carcinoma outcomes among First Nations Australians: A Strategic Plan for Action. Med J Aust 2024; 221: 230‐235. https://www.mja.com.au/journal/2024/221/5/overcoming‐disparities‐hepatocellular‐carcinoma‐outcomes‐first‐nations
- 13. Kudo M. Surveillance, diagnosis, and treatment outcome of hepatocellular carcinoma in Japan: 2023 update. Liver Cancer 2023; 12: 95‐102.
- 14. Hui S, Bell S, Le S, Dev A. Hepatocellular carcinoma surveillance in Australia: current and future perspectives. Med J Aust 2023; 219: 432‐438. https://www.mja.com.au/journal/2023/219/9/hepatocellular‐carcinoma‐surveillance‐australia‐current‐and‐future‐perspectives
- 15. Australian Institute of Health and Welfare. Cancer screening. Canberra: AIHW, 3 Dec 2024. https://www.aihw.gov.au/reports/australias‐health/cancer‐screening‐and‐treatment (viewed July 2024).
- 16. Schauer C, Mules T, van Rijnsoever M, Gane E. Increasing burden of advanced hepatocellular carcinoma in New Zealand‐the need for better surveillance. N Z Med J 2020; 133: 25‐34.
- 17. Allard N, Cabrie T, Wheeler E, et al. The challenge of liver cancer surveillance in general practice: do recall and reminder systems hold the answer? Aust Fam Physician 2017; 46: 859‐864.
- 18. Francica G, Borzio M. Status of, and strategies for improving, adherence to HCC screening and surveillance. J Hepatocell Carcinoma 2019; 6: 131‐141.
- 19. Flores JE, Thompson AJ, Hong T, et al. SAT‐496 Impacts of COVID on HCC care cascade recommendation concordance: maintained surveillance rates and improved linkage to care. J Hepatol 2024; 80: S402.
- 20. Kim YY, An C, Kim DY, et al. Failure of hepatocellular carcinoma surveillance: inadequate echogenic window and macronodular parenchyma as potential culprits. Ultrasonography 2019; 38: 311‐320.
- 21. Park H, Park JY. Clinical significance of AFP and PIVKA‐II responses for monitoring treatment outcomes and predicting prognosis in patients with hepatocellular carcinoma. Biomed Res Int 2013; 2013: 310427.
- 22. Li C, Zhang Z, Zhang P, Liu J. Diagnostic accuracy of des‐gamma‐carboxy prothrombin versus α‐fetoprotein for hepatocellular carcinoma: a systematic review. Hepatol Res 2014; 44: E11–25.
- 23. Guan MC, Zhang SY, Ding Q, et al. The performance of GALAD score for diagnosing hepatocellular carcinoma in patients with chronic liver diseases: a systematic review and meta‐analysis. J Clin Med Res 2023; 12: 949.
- 24. Yang JD, Addissie BD, Mara KC, et al. GALAD score for hepatocellular carcinoma detection in comparison with liver ultrasound and proposal of GALADUS score. Cancer Epidemiol Biomarkers Prev 2019; 28: 531‐538.
- 25. Chan HLY, Vogel A, Berg T, et al. A comparative analysis of Elecsys GALAD and Elecsys GAAD score to detect early‐stage hepatocellular carcinoma in an international cohort. Journal of Hepatology 2022; 77: S937.
- 26. Garay OU, Ambühl LE, Bird TG, et al. Cost‐effectiveness of hepatocellular carcinoma surveillance strategies in patients with compensated liver cirrhosis in the United Kingdom. Value Health 2024; 12: 1698‐1709.
- 27. Nan Y, Garay OU, Lu X, et al. Early‐stage hepatocellular carcinoma screening in patients with chronic hepatitis B in China: a cost‐effectiveness analysis. J Comp Eff Res 2024; 13: e230146.
- 28. Younossi ZM, Golabi P, Paik JM, et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 2023; 77: 1335‐1347.
- 29. Chouik Y, Aubin A, Maynard‐Muet M, et al. The grade of obesity affects the noninvasive diagnosis of advanced fibrosis in individuals with MASLD. Obesity (Silver Spring) 2024; 32: 1114‐1124.
- 30. Castellana M, Donghia R, Lampignano L, et al. Prevalence of the absence of cirrhosis in subjects with NAFLD‐associated hepatocellular carcinoma. J Clin Med 2021; 10: 4638.
- 31. Adams LA, Roberts SK, Strasser SI, et al. Nonalcoholic fatty liver disease burden: Australia, 2019–2030. J Gastroenterol Hepatol 2020; 35: 1628‐1635.
- 32. Hasegawa K, Takemura N, Yamashita T, et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2021 version (5th JSH‐HCC guidelines). Hepatol Res 2023; 53: 383‐390.
- 33. Chan MV, Huo YR, Trieu N, et al. Noncontrast MRI for hepatocellular carcinoma detection: a systematic review and meta‐analysis ‐ a potential surveillance tool? Clin Gastroenterol Hepatol 2022; 20: 44‐56.
- 34. ASHM. Viral Hepatitis Mapping Project [website]. ASHM Health, 2023. https://ashm.org.au/resources/viral‐hepatitis‐mapping‐project/ (viewed May 2024).
- 35. Gibson RN. Targeted liver ultrasound for chronic liver disease: time to focus? Australas J Ultrasound Med 2012; 15: 121‐125.
- 36. Singal AG, Llovet JM, Yarchoan M, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023; 78: 1922‐1965.
- 37. Piratvisuth T, Hou J, Tanwandee T, et al. Development and clinical validation of a novel algorithmic score (GAAD) for detecting HCC in prospective cohort studies. Hepatol Commun 2023; 7: e0317.
- 38. Garay O, Ambühl LE, Bird TG, et al. EE37 Cost‐utility analysis of Elecsys GAAD algorithm versus ultrasound plus ‐fetoprotein (AFP) for HCC surveillance in patients with compensated liver cirrhosis in the United Kingdom (UK). Value Health 2022; 25: S60.
- 39. Tzartzeva K, Obi J, Rich NE, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta‐analysis. Gastroenterology 2018; 154: 1706‐1718.
- 40. Department of Health and Aged Care. 4th National hepatitis B strategy 2023‐2030 [draft for consultation]. Canberra: Department of Health and Aged Care, 2023. https://www.hepatitisaustralia.com/Handlers/Download.ashx?IDMF=ac7e1443‐e999‐4927‐9284‐6dafe71f6a55 (viewed Aug 2024).






Open access:
Open access publishing facilitated by The University of Melbourne, as part of the Wiley – The University of Melbourne agreement via the Council of Australian University Librarians.
The authors thank Dr Cat Panwar, Panwar Health, for assistance with manuscript preparation.
Roche Diagnostics Australia provided funds for medical writing to support the development of the manuscript. Roche Diagnostics had no role in convening the authors or evaluating the content, nor placed any restrictions regarding the submission of the manuscript for publication. Jacob George is supported by the Robert W. Storr Bequest to the Sydney Medical Foundation, University of Sydney; a National Health and Medical Research Council of Australia (NHMRC) Program Grant (APP1053206), Investigator and Medical Research Future Fund grants (APP2032407; NCRI000183; APP2016215; APP 2010795; APP1196492) and a Cancer Institute, NSW grant (2021/ATRG2028). He is on several advisory boards and receives honoraria for talks from Novo Nordisk, Astra Zeneca, Roche, BMS, Pfizer, Cincera, Pharmaxis, Boehringer Mannheim, CSL, Gilead and Eisai. Jacob George, Jess Howell and Alexander Thompson are involved in establishing pilot studies of GAAD, which is a test being developed by Roche Diagnostics.
Author contributions:
Howell J: Conceptualization, writing – original draft, writing – review and editing. Emery J: Writing – review and editing. Roberts S: Writing – review and editing. Thompson A: Writing – review and editing. Ng M: Writing – review and editing. George J: Writing – review and editing. Leggett B: Writing – review and editing. Tse E: Writing – review and editing. Nguyen B: Writing – review and editing. Combo T: Writing – review and editing. Wigg A: Writing – review and editing.