The known: Preoperative breast MRI has higher sensitivity than conventional imaging, but little evidence confirms which patient subgroups are likely to benefit.
The new: MRI was most frequently requested for women with dense breasts. There was an absolute increase in mastectomy of 13 percentage points following MRI, and increases were seen for all subgroups except women aged ≥ 70 years and those for whom neoadjuvant therapy was already planned. The majority of changes in surgery plans (85%) were potentially justified by the final pathology findings.
The implications: MRI for selected women where conventional imaging is suboptimal may improve surgical planning and thus afford better outcomes. MRI is less likely to change outcomes in older women.
Breast‐conserving surgery (BCS) is the commonest surgical procedure in female patients with early breast cancer,1 providing similar survival outcomes to mastectomy when combined with radiotherapy.2 About 20–30% of women undergo further surgery due to involved or close surgical margins.3,4,5 The ability to offer BCS depends on preoperative imaging confirming tumour extent. Conventional imaging (mammography, ultrasound) offers good anatomical information; however, contrast‐enhanced imaging may be better for locally staging the index cancer for optimal surgery.6
Magnetic resonance imaging (MRI) has superior sensitivity compared with mammography and/or ultrasound,7,8,9 detecting additional cancer foci in about 16% of women,10 and detecting contralateral disease.11 However, there has been concern that the sensitivity of MRI may increase mastectomy rates with uncertain clinical benefit.10 Randomised controlled trials (RCTs) of preoperative MRI have not consistently shown improved surgical outcomes in unselected women, and it is unclear which subpopulations may benefit.12,13,14,15
Internationally, studies including the Multicenter International Prospective Analysis (MIPA) trial16 address whether and how MRI affects breast cancer surgery. Preoperative staging MRI was more likely to be used in younger women, those with lobular pathology, dense breasts or larger tumours, and those for whom mastectomy had been planned based on conventional imaging (22.4% in the MRI group versus 14.4% in the no MRI group).16 MRI led to conversion from BCS to mastectomy in 11.6% of women (prompted by additional MRI findings in 9.1%) and mastectomy to BCS in 0.3% of women. Compared with women who had conventional staging, those in the MRI group had a lower reoperation rate (8.5% versus 11.7%). Meta‐analyses suggest that preoperative MRI in unselected women may increase mastectomy rates,17 but have also shown that study findings vary in terms of the effects of preoperative MRI on local and distant recurrence‐free survival and other surgical outcomes,18,19 and that preoperative MRI might lead to fewer re‐excisions for lobular cancers.20
The effect of MRI in defined clinical scenarios at the time of cancer diagnosis is unclear21,22 and access in many settings is limited. In Australia, government funding by Medicare (items 63533 and 63534) is contingent on further Australian data showing whether and in whom MRI may improve treatment planning and outcomes, with the Medicare rebate for breast MRI limited to specialists.23
To address this uncertainty, we undertook a prospective, multicentre study to describe reasons why preoperative MRI is requested in Australia and the associated changes in treatment that occur, with the aim of characterising patient subpopulations that may benefit.
Methods
Study design and eligibility criteria
This was a multicentre prospective observational study of systematically collected data on consecutively recruited women with newly diagnosed breast cancer, for whom the local multidisciplinary team (of at least a surgeon and a radiologist) recommended breast MRI to better plan treatment, as per usual documented practice. The study was registered prospectively with the Australian New Zealand Clinical Trials Registry (ACTRN12620000282987; 3 March 2020). We report our study according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Supporting Information).24
Recruitment took place over 23 months (15 September 2020 to 14 July 2022) at seven centres in Australia (Mater Hospital and Bankstown–Lidcombe Hospital in Sydney; Royal Melbourne Hospital and St Vincent's Hospital in Melbourne; Fiona Stanley Hospital, Royal Perth Hospital and St John of God Subiaco Hospital in Perth), with 43 surgeons participating. Eligible patients included those for whom the treating team deemed MRI would aid treatment planning for one or more of the following reasons: ultrasound, mammography and/or clinical examination results were discrepant in size or focality; the woman was younger than 70 years and had invasive lobular cancer; the woman was younger than 50 years; and the woman had mammographically dense (Breast Imaging Reporting and Data System category C or D) breasts. Patients were excluded if they: had distant metastases; had locally advanced inoperable cancer; had previously had cancer on the same side; had classical lobular carcinoma in situ; had other non‐malignant systemic diseases that would prevent breast surgery with curative intent; had undergone MRI before registration; or were unable to undergo MRI. Women recommended for neoadjuvant systemic therapy before or after final imaging assessment were not excluded. Patients had conventional imaging, and may have had three‐dimensional or contrast‐enhanced mammography. As the very rare cases of breast cancer in male patients almost always undergo mastectomy, the target recruitment population for this study was women with breast cancer (biological females and others identifying as women).
MRI procedures
Contrast‐enhanced MRI was performed on a 1.5 Tesla or 3 Tesla machine, using dedicated breast coils and site‐specific protocols (full diagnostic or abbreviated) that met the technical recommendations of the American College of Radiology. The intravenous contrast agent used was gadobutrol (Gadovist, Bayer Group), which was given at a dose of 0.1 mL/kg at 2–3 mL per second, using a power injector, and followed by a saline flush.
Data collection
Patient demographic data, the reason(s) for requesting MRI and a pre‐MRI treatment plan completed by the multidisciplinary team were recorded at recruitment. Demographic data included age, body mass index, country of birth, primary language, and socio‐economic status (derived from the Australian Bureau of Statistics Index of Relative Socio‐economic Advantage and Disadvantage25). Multiple reasons for ordering MRI could be recorded. The pre‐MRI treatment plan included: planned breast and axillary surgery; expected radiotherapy fields; and probable systemic therapy (including neoadjuvant chemotherapy). The treatment plan was reviewed and recorded after MRI results were available, by the multidisciplinary team. The primary outcome was change in surgical treatment plan after MRI.
A clinical review of planned treatment, locally reported imaging results, delivered treatment and final pathology reports was undertaken by the senior investigator (CMS), supported by other authors, to assess whether final pathology findings justified changes in surgical treatment. Changes could be from an initial pre‐MRI plan of less extensive surgery to more extensive surgery (from BCS to BCS plus oncoplasty, from BCS or BCS plus oncoplasty to mastectomy, or from unilateral to bilateral mastectomy) or from more extensive surgery to less extensive surgery. The three criteria used to classify a change as justified were: diagnosis of proven contralateral cancer found on MRI necessitating bilateral (more extensive) surgery; diagnosis of multifocal or extensive cancer on MRI but not initial imaging, and confirmed by final pathology as > 4 cm of tumour and/or in > 1 quadrant, with these features assuming justification of more extensive surgery; and diagnosis of less extensive cancer on MRI, and confirmed on final pathology, justifying less extensive surgery. In addition, the number of re‐excisions for close or involved margins within 12 months of initial surgery were recorded. Further, patient‐reported outcome data were recorded, but these will be reported separately.
Data analysis and synthesis
The a priori target sample size was 400 women to estimate a 15 percentage point difference between the pre‐MRI and post‐MRI proportions of women for whom mastectomy or BCS was planned, with an absolute precision of 4 percentage points. Participant characteristics were summarised as mean (standard deviation [SD]) or median (interquartile range) values for continuous variables, and as proportions and 95% exact confidence intervals for categorical variables. Differences in age between state‐based recruitment sites were tested with analysis of variance (ANOVA). Specific treatment plan proportions (subclassified under breast surgery, axillary surgery, radiotherapy and systemic therapy) were compared before and after MRI with McNemar's test. Generalised linear regression (PROC GENMOD with the REPEATED statement, binomial distribution and identity link in SAS) was used to compare pre‐MRI versus post‐MRI mastectomy treatment plan proportions, estimated as absolute risk differences (RDs) with Wald 95% confidence intervals. Interaction terms were included to explore changes by state and age group (< 40 years; 40–49 years; 50–59 years; 60–69 years; ≥ 70 years). Multiple reasons for ordering MRI could be recorded per participant; hence, separate models were conducted to investigate the change in proportion for each reason.
The proportions of changes in surgical management that were deemed justified by final pathology findings were computed. Changes were classified as either to less extensive surgery or to more extensive surgery, and the proportions of justified changes were compared using Fisher's exact test. Justification for mastectomy was assessed by absolute tumour size > 4 cm and by multifocality and centricity.
All tests of statistical significance were two‐sided. The level chosen for statistical significance was P < 0.05. Analyses were conducted in SAS version 9.4 (SAS Institute) and Stata version 18.0 (StataCorp).
Ethics approval
This research was approved by the Western Australian Department of Health, South Metropolitan Health Service Human Research Ethics Committee (RGS0003657). All participants provided written consent prior to entering the study.
Results
Participant characteristics
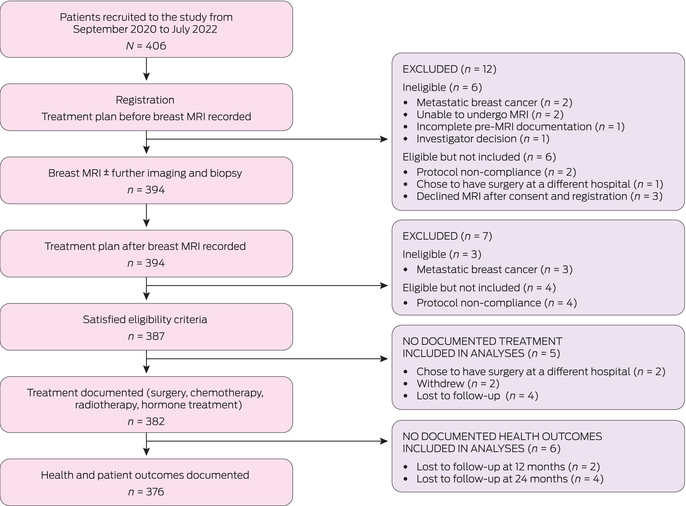
A total of 406 participants were recruited and followed for 2 years ± 6 months. After exclusions, 387 women had both pre‐MRI and post‐MRI documented treatment plans (Box 1). Participant characteristics are described in Box 2. Most participants were enrolled in New South Wales (155 [40%]) or Victoria (158 [41%]), with the remainder recruited in Western Australia (74 [19%]). Mean participant age was 54.9 years (SD, 10.6 years); participants in WA were significantly younger (mean age, 50.5 years [SD, 10.7 years]) than in NSW (mean age, 56.1 years [SD, 11.5 years]) and Victoria (mean age, 55.9 years [SD, 8.9 years]) (overall ANOVA, P < 0.001).
Reasons for requesting MRI
Reasons for requesting MRI were recorded from predefined criteria, with multiple reasons permitted per participant. Overall, high breast density was the most recorded reason (252/387 women [65%]), followed by size discrepancy in prior imaging or clinical assessment (161 [42%]), multifocality (108 [28%]) and young age (< 50 years) (105 [27%]) (Box 2). Consistent with overall younger mean age, young age was a more frequent reason for ordering MRI in WA (36/74 [49%]) compared with NSW (53/155 [34%]) and Victoria (33/158 [21%]). Size discrepancy and multifocality were less frequently recorded reasons in Victoria (20 [13%] and 28 [18%], respectively) than in NSW (106 [68%] and 51 [33%]) and WA (35 [47%] and 29 [39%]).
Changes in surgical treatment plan
Overall, the treatment plan was changed after MRI assessment for 198 participants (51% [95% CI, 46–56%]), including changes in type of surgery, radiotherapy, systemic therapy, and combinations thereof (Box 3). In total, 119 participants (31% [95% CI, 26–36%]) had a change in breast surgery treatment plan. There was a significant increase in the proportion of participants with any planned mastectomy (unilateral or bilateral), from 57 before MRI (15%) to 107 after MRI (28%) (RD, 13 percentage points [95% CI, 9–17]; P < 0.001) (Box 4), including increases in unilateral mastectomy (RD, 10 percentage points [95% CI, 6–14]; P < 0.001) and bilateral mastectomy (RD, 3 percentage points [95% CI, 1–5]; P < 0.001) (Box 3). Concomitantly, there was a significant decrease in participants with planned BCS, from 269 (70%) before MRI to 219 (57%) after MRI, with the difference mirroring the change in mastectomy rate (RD, –13 percentage points [95% CI, –17 to 8]; P < 0.001) (Box 3). There was no evidence of a difference in plans for oncoplastic breast surgery (defined as volume displacement or replacement > 20%) (Box 3).
Changes in mastectomy by age, state and MRI reason
Descriptive stratified analyses by age group, state and type of planned surgery are shown in Box 4. Increases in any mastectomy recommendation after MRI were observed for age groups < 40 years (RD, 10 percentage points [95% CI, –10 to 30]), 40–49 years (RD, 18 percentage points [95% CI, 10 to 27]), 50–59 years (RD, 10 percentage points [95% CI, 4 to 16]) and 60–69 years (RD, 19 percentage points [95% CI, 9–29]) (Box 4, Box 5). There was no evidence of an increase in mastectomy recommendation after MRI in women aged ≥ 70 years (RD, –3 percentage points [95% CI, –15 to 9]). The same pattern of results was generally observed for unilateral mastectomy only (Box 4).
There was an increase in mastectomy recommendation after MRI in all states, but the magnitude varied. Similar increases were observed in NSW (RD, 10 percentage points [95% CI, 3 to 17]) and Victoria (RD, 8 percentage points [95% CI, 3 to 13]), with a larger increase in WA (RD, 30 percentage points [95% CI, 18 to 41]). Results for unilateral mastectomy and for any mastectomy (unilateral and bilateral combined) were similar (Box 4).
Increases in mastectomy recommendation were observed for all reasons for requesting MRI except pre‐MRI planned neoadjuvant therapy (Box 4). The largest increases were for size discrepancy (any mastectomy RD, 17 percentage points [95% CI, 9 to 25]; unilateral mastectomy RD, 14 percentage points [95% CI, 6 to 22]) and multifocality (any mastectomy RD, 16 percentage points [95% CI, 6 to 26]; unilateral mastectomy RD, 13 percentage points [95% CI, 3 to 23]). There was no evidence of an increase in unilateral mastectomy in patients with planned neoadjuvant therapy (RD, 2 percentage points [95% CI, –11 to 14]).
Appropriateness of changes in surgical plan
For 88 patients with a change in surgical management (excluding 31 patients who received neoadjuvant therapy before surgery or had a change in axillary surgery only), final pathology findings were reviewed to assess whether the change was justified. Based on clinical review, the change in management was deemed justified in 75 patients (85% [95% CI, 75–91%]). Box 6 describes the changes in surgical management after MRI; a change to more extensive surgery (73/88 women; 83% [95% CI, 74–90%]) was more frequent than a change to less extensive surgery (15/88 women; 17% [95% CI, 10–27%]). The proportion of justified changes was larger in the less extensive group (15/15; 100% [95% CI, 78–100%]) than in the more extensive group (60/73; 82% [95% CI, 71–90%]), although this difference was not statistically significant (P = 0.11) suggesting MRI accurately predicted who could avoid mastectomy in all our cases.
Re‐excision
The overall re‐excision rate was 14% (95% CI, 11–18%) (53 of the 379 women who had breast surgery). Of these 53 patients, 35 (66% [95% CI, 52–79]) had a single re‐excision and still achieved breast conservation, but the remaining 18 (34% [95% CI, 22–48%]) underwent mastectomy as second, third or fourth surgery. The re‐excision rate was comparable at 15% (95% CI, 8–24%; 13/88) in those for whom a change in surgical treatment was made following MRI, irrespective of whether this change was deemed justified or not.
Discussion
Preoperative breast MRI has the potential to improve outcomes for selected women with breast cancer by detecting additional disease and leading to changes to more appropriate treatments. Primarily, it may be useful for optimising surgery to reduce the risks of missed cancer and of needing re‐excision due to involved margins. Despite this, the role of MRI in improving outcomes has been unclear, with evidence from RCTs and meta‐analyses variable, and concern about the potential for an unfavourable benefit–harm ratio and a poor cost trade‐off from increases in mastectomy after MRI. To our knowledge, we report the first analysis of Australian data describing patients with predefined criteria for whom MRI was deemed useful by the treating clinical team, along with associated changes in planned management. High breast density was the most frequently reported reason for requesting MRI, usually with an additional reason; other common reasons included a size discrepancy on prior workup, multifocality and young age. A change in breast surgery occurred in about one‐third of patients, with an absolute increase in mastectomy (and decrease in BCS) of 13 percentage points. There was no observed increase in mastectomy in older women (aged ≥ 70 years) or women for whom neoadjuvant therapy was planned. Based on clinical review, the majority (85%) of surgical changes were deemed justifiable by final pathology findings. As expected, MRI did not change plans for axillary surgery (determined by preoperative pathology findings that confirm involved nodes) or systemic treatment (determined by tumour biology).
The magnitude of increase in mastectomy observed in our study (13 percentage points) is consistent with international findings such as the MIPA trial, in which an 11.3 percentage point increase in mastectomies was reported in the MRI group,16 and is comparable to the range of reported study‐level increases (increases of 1.4 to 16.2 percentage points in 14 studies).17 RCTs have failed to show any clinical utility in MRI in all‐comers — that is, any women with newly diagnosed early breast cancer but without a specific reason to believe conventional imaging is inadequate to locally stage the disease.12,14,15 Our study targeted only patients for whom the treating clinical team believed MRI may be useful, and indeed demonstrates that MRI has clinical utility in the subgroups of patients currently recommended this — those with clinical or conventional imaging discrepancy or dense breasts, young women, and those with lobular or multifocal cancers. Although the numbers were small, it is notable that MRI does not seem to change surgical outcomes for women aged 70 years or older. The fact that MRI did not change mastectomy recommendations in those undergoing neoadjuvant chemotherapy was expected, as these women often have a predicted excellent response to treatment that depends on cancer biology rather than anatomical size. MRI may provide useful information regarding response to neoadjuvant chemotherapy, but assessing this was not the purpose of this study.
Of interest from our data was the lack of change from BCS to oncoplasty. This may have been an expected result if MRI findings had shown more extensive disease, and is worthy of further study.
The re‐excision rate in our study is lower than that reported in 2018 national breast surgery audit data,5 but accords with current audit data from some of the current study institutions (unpublished data, Christobel Saunders).
Studies that assess changes in management are surrogates for potential improvements in patient outcomes. Implicit in inferences about improved outcomes are assumptions that a new test (ie, MRI) improves accuracy of management decisions and thereby leads to clinical benefit.26 RCTs are the ideal type of study to evaluate this, but they may be cumbersome, expensive and impractical.27 Assessing the impact of better diagnostic tools on long term cancer outcomes is challenging, so conducting before–after studies such as this, enrolling consecutive patients with a well defined question, is a pragmatic alternative that allows changes in planned management to be quantified.27 We also included an assessment of the “correctness” of information provided by MRI to enhance inferences about potential clinical benefit.26,27 Our finding that most surgical changes were justified accords with findings that have demonstrated MRI's accuracy in detecting additional cancer foci and contralateral disease.28
Consistent with international data,16 MRI is most frequently used in women with high breast density and those who are young.29 The current Medicare rebate refers to patients in whom “there is a discrepancy between the clinical assessment and the conventional imaging assessment of the extent of the malignancy” and “the results of breast MRI imaging may alter treatment planning”.30 Our study contributes to defining the role of MRI in improving outcomes in subgroups of patients at high risk of recurrence, including those younger than 70 years with dense breasts, lobular cancer, multifocal cancer and/or clinical and radiological disparity in tumour extent. Without long term follow‐up and a larger dataset, the effects of MRI on cancer recurrence will remain unknown.
In the time since our trial began, the PROSPECT trial of women aged 50 years or older with clinical early stage cancer was published.31 Its findings indicate that MRI may also be useful for identifying women with unifocal cancer in whom radiotherapy may be safely omitted, suggesting potentially important roles of preoperative MRI beyond that investigated in our study.
Limitations
We did not include a comparison group who did not have MRI, but data from contemporaneous patients in participating institutions who did not receive MRI are being evaluated and will be reported; however, patient and tumour characteristics are likely to vary between those who did and did not have MRI. An inherent limitation in diagnostic before–after study designs is that the pre‐test management plan is somewhat hypothetical27 so it may differ if MRI is not planned or not available. Increasing use of contrast mammography may dilute some advantages of MRI, although we do not yet know which women would benefit more from which modality. Finally, although final pathology findings were carefully reviewed against defined criteria for the justification of a change in surgery, this was performed retrospectively and draws on clinical judgement. Prospective data collection and independent review by multiple clinicians would have strengthened our findings.
Conclusion
Preoperative MRI led to changes in surgical plans in about a third of selected women with operable early breast cancer, with an increase in mastectomy rate of 13 percentage points. In most cases, changes were appropriate, but for some individuals MRI may lead to unnecessarily extensive surgery.
Box 2 – Descriptive statistics for all patients
|
|
Number (%)* |
||||||||||||||
|
|
|||||||||||||||
|
Total number participants |
387 |
||||||||||||||
|
Characteristics |
|
||||||||||||||
|
Age in years, mean (SD) |
54.9 (10.6) |
||||||||||||||
|
BMI in kg/m2, median (IQR) |
25.7 (22.5–29.8) |
||||||||||||||
|
Country of birth |
|
||||||||||||||
|
Australia |
230 (59.4%) |
||||||||||||||
|
Overseas |
157 (40.6%) |
||||||||||||||
|
Main language spoken |
|
||||||||||||||
|
English |
356 (92.0%) |
||||||||||||||
|
Other |
31 (8.0%) |
||||||||||||||
|
IRSD quintile |
|
||||||||||||||
|
Q1 (most disadvantaged) |
30 (7.8%) |
||||||||||||||
|
Q2 |
43 (11%) |
||||||||||||||
|
Q3 |
51 (13%) |
||||||||||||||
|
Q4 |
83 (21%) |
||||||||||||||
|
Q5 (least disadvantaged) |
180 (46.5%) |
||||||||||||||
|
State (institution location) |
|
||||||||||||||
|
New South Wales |
155 (40.1%) |
||||||||||||||
|
Victoria |
158 (40.8%) |
||||||||||||||
|
Western Australia |
74 (19%) |
||||||||||||||
|
Reason for MRI † |
|
||||||||||||||
|
Young age |
105 (27.1%) |
||||||||||||||
|
Size discrepancy |
161 (41.6%) |
||||||||||||||
|
Dense breasts |
252 (65.1%) |
||||||||||||||
|
Multifocality |
108 (27.9%) |
||||||||||||||
|
Lobular cancer |
71 (18%) |
||||||||||||||
|
Mutation carrier |
3 (1%) |
||||||||||||||
|
Neoadjuvant therapy planned |
63 (16%) |
||||||||||||||
|
Patient request |
1 (0.3%) |
||||||||||||||
|
|
|||||||||||||||
|
BMI = body mass index. IQR = interquartile range. IRSD = Index of Relative Socio‐economic Disadvantage. MRI = magnetic resonance imaging. SD = standard deviation. * Data are number (%) unless otherwise specified. † Total does not sum to 100% because multiple reasons could be specified. |
|||||||||||||||
Box 3 – Pre‐MRI and post‐MRI treatment plans
|
|
Number (%)* |
|
|||||||||||||
|
|
Pre‐MRI |
Post‐MRI |
P |
||||||||||||
|
|
|||||||||||||||
|
Total number of participants |
387 |
387 |
|
||||||||||||
|
Breast surgery |
|
|
|
||||||||||||
|
Unilateral mastectomy |
53 (14%) |
91 (24%) |
< 0.001 |
||||||||||||
|
Bilateral mastectomy |
4 (1%) |
16 (4.1%) |
< 0.001 |
||||||||||||
|
Breast conserving surgery |
269 (69.5%) |
219 (56.6%) |
< 0.001 |
||||||||||||
|
Breast conserving surgery with level 2 oncoplastic technique |
59 (15%) |
64 (17%) |
0.63 |
||||||||||||
|
Axillary surgery |
|
|
|
||||||||||||
|
Axillary clearance |
32 (8.3%) |
42 (11%) |
0.02 |
||||||||||||
|
Sentinel lymph node biopsy |
310 (80.1%) |
309 (79.8%) |
> 0.99 |
||||||||||||
|
Other† |
9 (2%) |
12 (3.1%) |
0.58 |
||||||||||||
|
Radiotherapy |
|
|
|
||||||||||||
|
Chest wall |
29 (7.5%) |
40 (10%) |
0.08 |
||||||||||||
|
Breast |
319 (82.4%) |
272 (70.3%) |
< 0.001 |
||||||||||||
|
Regional nodes |
33 (8.5%) |
41 (11%) |
0.12 |
||||||||||||
|
Other‡ |
7 (2%) |
1 (0.3%) |
0.03 |
||||||||||||
|
Systemic therapy |
|
|
|
||||||||||||
|
Chemotherapy |
86 (22%) |
84 (22%) |
0.88 |
||||||||||||
|
Human epidermal growth factor receptor 2 directed |
33 (8.5%) |
35 (9.0%) |
0.77 |
||||||||||||
|
Endocrine |
263 (68.0%) |
280 (72.4%) |
0.01 |
||||||||||||
|
Neoadjuvant |
66 (17%) |
81 (21%) |
0.01 |
||||||||||||
|
Other§ |
10 (2.6%) |
5 (1%) |
0.18 |
||||||||||||
|
|
|||||||||||||||
|
MRI = magnetic resonance imaging. * Data are number (%) unless otherwise specified. † Targeted axillary dissection; targeted axillary dissection and sentinel node biopsy; targeted axillary dissection and fine needle aspiration, pending further investigations; left breast axillary clearance; bilateral. ‡ Pending further investigations; supraclavicular fossa; radiotherapy to breast and regional nodes. § Pending further investigations; immunotherapy (Neo‐N trial). |
|||||||||||||||
Box 4 – Absolute differences between pre‐MRI and post‐MRI changes in mastectomy proportion by age group, state and reason for ordering MRI
|
|
Total number of patients |
Number of patients (% [95% CI]) for whom mastectomy was planned before MRI |
Number of patients (% [95% CI]) for whom mastectomy was planned after MRI |
Percentage point difference (95% CI) (pre‐MRI v post‐MRI) |
|||||||||||
|
|
|||||||||||||||
|
Any mastectomy (unilateral or bilateral) |
387 |
57 (15% [11% to 18%]) |
107 (27.6% [23.2% to 32.1%]) |
13 (8.7 to 17) |
|||||||||||
|
By age group |
|
|
|
|
|||||||||||
|
< 40 years |
29 |
9 (31% [14% to 48%]) |
12 (41% [24% to 59%]) |
10 (–10 to 30) |
|||||||||||
|
40–49 years |
93 |
17 (18% [10% to 26%]) |
34 (37% [27% to 46%]) |
18 (10 to 27) |
|||||||||||
|
50–59 years |
146 |
19 (13% [7.6% to 18%]) |
34 (23% [16% to 30%]) |
10 (4.4 to 16) |
|||||||||||
|
60–69 years |
83 |
7 (8% [3% to 14%]) |
23 (28% [18% to 37%]) |
19 (9.0 to 29) |
|||||||||||
|
≥ 70 years |
36 |
5 (14% [3% to 25%]) |
4 (11% [0 to 21%]) |
–3 (–15 to 9) |
|||||||||||
|
By state |
|
|
|
|
|||||||||||
|
New South Wales |
155 |
(17% [11% to 23%]) |
41 (26% [20% to 33%]) |
10 (2.6 to 17) |
|||||||||||
|
Victoria |
158 |
13 (8% [3.9% to 13%]) |
26 (16% [11% to 22%]) |
8.2 (3.3 to 13) |
|||||||||||
|
Western Australia |
74 |
18 (24% [15% to 34%]) |
40 (54% [43% to 65%]) |
30 (18 to 41) |
|||||||||||
|
By MRI reason |
|
|
|
|
|||||||||||
|
Size discrepancy |
161 |
29 (18% [12% to 24%]) |
57 (35% [28% to 43%]) |
17 (9.4 to 25) |
|||||||||||
|
Density |
252 |
39 (15% [11% to 20%]) |
65 (26% [20% to 31%]) |
10 (5.6 to 15) |
|||||||||||
|
Multifocality |
108 |
28 (26% [18% to 34%]) |
45 (42% [32% to 51%]) |
16 (5.7 to 26) |
|||||||||||
|
Lobular histology |
71 |
12 (17% [8.2% to 26%]) |
19 (27% [17% to 37%]) |
10 (2 to 18) |
|||||||||||
|
Neoadjuvant therapy |
63 |
21 (33% [22% to 45%]) |
22 (35% [23% to 47%]) |
2 (–11 to 14) |
|||||||||||
|
Unilateral mastectomy |
387 |
53 (14% [10% to 17%]) |
91 (24% [19% to 28%]) |
9.8 (5.6 to 14) |
|||||||||||
|
By age group |
|
|
|
|
|||||||||||
|
< 40 years |
29 |
8 (28% [11% to 44%]) |
9 (31% [14% to 48%]) |
3 (–17 to 24) |
|||||||||||
|
40–49 years |
93 |
15 (16% [8.7% to 24%]) |
27 (29% [20% to 38%]) |
13 (4.4 to 21) |
|||||||||||
|
50–59 years |
146 |
18 (12% [7.0% to 18%]) |
28 (19% [13% to 26%]) |
6.8 (1.3 to 12) |
|||||||||||
|
60–69 years |
83 |
7 (8% [3% to 14%]) |
23 (28% [18% to 37%]) |
19 (9.0 to30) |
|||||||||||
|
≥ 70 years |
36 |
5 (14% [3% to 25%]) |
4 (11% [1% to 21%]) |
–3 (–15 to 9) |
|||||||||||
|
By state |
|
|
|
|
|||||||||||
|
New South Wales |
155 |
26 (17% [11% to 23%]) |
38 (25% [18% to 31%]) |
7.7 (1.0 to 15) |
|||||||||||
|
Victoria |
158 |
13 (8.2% [3.9% to 13%]) |
23 (15% [9.1% to 20%]) |
6.3 (1.5 to 11) |
|||||||||||
|
Western Australia |
74 |
14 (19% [10% to 28%]) |
30 (41% [29% to 52%]) |
22 (9.0 to 34) |
|||||||||||
|
By MRI reason |
|
|
|
|
|||||||||||
|
Size discrepancy |
161 |
26 (16% [10% to 22%]) |
49 (30% [23% to 38%]) |
14 (6.6 to 22) |
|||||||||||
|
Density |
252 |
37 (15% [10% to 19%]) |
56 (22% [17% to 27%]) |
7.5 (2.7 to 12) |
|||||||||||
|
Multifocality |
108 |
27 (25% [17% to 33%]) |
41 (38% [29% to 47%]) |
13 (3.0 to 23) |
|||||||||||
|
Lobular histology |
71 |
11 (15% [7.1% to 24%]) |
18 (25% [15% to 35%]) |
10 (2 to 18) |
|||||||||||
|
Neoadjuvant therapy |
63 |
21 (33% [22% to 45%]) |
22 (35% [23% to 47%]) |
2 (–11 to 14) |
|||||||||||
|
|
|||||||||||||||
|
MRI = magnetic resonance imaging. |
|||||||||||||||
Box 5 – Pre‐MRI versus post‐MRI surgical plans by type of surgery and age group
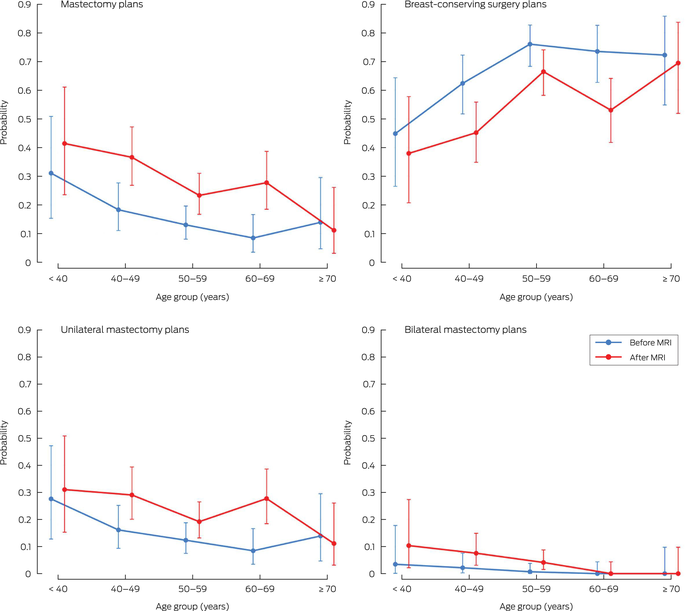
MRI = magnetic resonance imaging.
Box 6 – Surgical changes justified by pathology findings
|
|
|
Change in surgery justified by pathology findings |
|||||||||||||
|
|
Total number of patients with change |
Number of patients with justified change |
Percentage of patients with justified change (95% CI) |
||||||||||||
|
|
|||||||||||||||
|
All surgical changes after MRI |
88 |
75 |
85% (75–91%) |
||||||||||||
|
Less extensive surgery after MRI |
15 |
15 |
100% (78–100%) |
||||||||||||
|
BCS and oncoplasty to BCS |
6 |
6 |
100% (54–100%) |
||||||||||||
|
Unilateral mastectomy to BCS |
4 |
4 |
100% (40–100%) |
||||||||||||
|
Unilateral mastectomy to BCS and oncoplasty |
5 |
5 |
100% (48–100%) |
||||||||||||
|
More extensive surgery after MRI |
73 |
60 |
82% (71–90%) |
||||||||||||
|
BCS to BCS and oncoplasty |
23 |
18 |
78% (56–93%) |
||||||||||||
|
BCS to unilateral mastectomy |
34 |
29 |
85% (69–95%) |
||||||||||||
|
BCS and oncoplasty to unilateral mastectomy |
11 |
10 |
91% (59–100%) |
||||||||||||
|
BCS and oncoplasty to bilateral mastectomy |
1 |
1 |
100% (3–100%) |
||||||||||||
|
Unilateral mastectomy to bilateral mastectomy |
4 |
2 |
50% (7–93%) |
||||||||||||
|
|
|||||||||||||||
|
BCS = breast‐conserving surgery. MRI = magnetic resonance imaging. |
|||||||||||||||
Received 28 February 2025, accepted 18 July 2025
- Michael L Marinovich1
- Nehmat Houssami1,2
- Andrew Spillane2,3
- Gregory B Mann4,5,6
- Donna Taylor7,8
- Michelle Reintals9,10
- Nadine Phillips4,11
- Max K Bulsara11
- Patsy Siok Hwa Soon12,13
- Tracey Dickens8
- Christobel M Saunders4,8
- 1 Daffodil Centre, University of Sydney and Cancer Council NSW, Sydney, NSW
- 2 University of Sydney, Sydney, NSW
- 3 Melanoma Institute Australia, Sydney, NSW
- 4 University of Melbourne, Melbourne, VIC
- 5 Royal Melbourne Hospital, Melbourne, VIC
- 6 Royal Women's Hospital, Melbourne, VIC
- 7 Royal Perth Hospital, Perth, WA
- 8 University of Western Australia, Perth, WA
- 9 BreastScreen SA, Adelaide, SA
- 10 University of Adelaide, Adelaide, SA
- 11 Institute for Health Research, University of Notre Dame Australia, Fremantle, WA
- 12 Bankstown‐Lidcombe Hospital, Sydney, NSW
- 13 UNSW Sydney, Sydney, NSW
Open access:
Open access publishing facilitated by The University of Melbourne, as part of the Wiley – The University of Melbourne agreement via the Council of Australian University Librarians.
Data Sharing:
The data that underlie this report are available for sharing. Enquiries should be directed to the corresponding author.
Author contributions:
Marinovich ML: Formal analysis, writing – original draft, writing – review and editing. Houssami N: Methodology, writing – review and editing. Spillane A: Investigation, writing – review and editing. Mann GB: Investigation, writing – review and editing. Taylor D: Investigation, writing – review and editing. Reintals M: Investigation, writing – review and editing. Phillips N: Formal analysis, writing – review and editing. Bulsara MK: Methodology, writing – review and editing. Soon P: Investigation, writing – review and editing. Dickens T: Project administration, writing – review and editing. Saunders CM: Conceptualization, funding acquisition, supervision, methodology, writing – review and editing.
This study was supported by a grant from the Medical Research Future Fund's (Australian Government Department of Health, Disability and Ageing) Targeted Health System and Community Organisation Research initiative (MRF1177121). The funder had no role in study design, data collection, analysis or interpretation, reporting, or publication. We thank Jocelyn Lippey, Saud Hamza and Helen Ballal for coordinating recruitment at their respective study sites, and Breast Cancer Trials for providing data management services.
Michael Marinovich and Nehmat Houssami received funding from the National Breast Cancer Foundation (2023/IIRS0028 and EC‐21‐001) paid to their institutions. Nehmat Houssami received funding from the National Health and Medical Research Council (1194410) paid to her institution, and is a member of the BreastScreen Australia National Policy Review Expert Advisory Group 2023–2025. Andrew Spillane, Patsy Soon, Tracey Dickens and Christobel Saunders received funding from the Medical Research Future Fund (MRF1177121) for this study, paid to their institutions. Andrew Spillane has been paid speaker fees from Eli Lilly Australia and The Limbic, and is Deputy Chair of the Board of Breast Cancer Trials. Donna Taylor is a member of the Breast Imaging Advisory Committee of the Royal Australian and New Zealand College of Radiologists.
- 1. Agarwal S, Pappas L, Neumayer L, et al. Effect of breast conservation therapy vs mastectomy on disease‐specific survival for early‐stage breast cancer. JAMA Surgery 2014; 149: 267‐274.
- 2. Fisher B, Anderson S, Bryant J, et al. Twenty‐year follow‐up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347: 1233‐1241.
- 3. Marinovich ML, Saunders CM, Pereira G, Houssami N. Rates of reoperation after breast conserving cancer surgery in Western Australia before and after publication of the SSO‐ASTRO margins guideline. Breast 2023; 69: 499‐505.
- 4. van Leeuwen MT, Falster MO, Vajdic CM, et al. Reoperation after breast‐conserving surgery for cancer in Australia: statewide cohort study of linked hospital data. BMJ Open 2018; 8: e020858.
- 5. Royal Australian College of Surgeons. BreastSurgANZ Quality Audit: annual report 2018. Version 2.0. Melbourne: Breast Surgeons of Australia and New Zealand, 2020. https://www.surgeons.org/‐/media/Project/RACS/surgeons‐org/files/morbidity‐audits/BQA_Annual_Report_2018.pdf?rev=e335c65f7d7545f7b853abe5fd058f98&hash=D2902C19C26030651C56C787F76D3617 (viewed Feb 2025).
- 6. Mann RM, Cho N, Moy L. Breast MRI: state of the art. Radiology 2019; 292: 520‐536.
- 7. Sardanelli F, Podo F, Santoro F, et al; for the High Breast Cancer Risk Italian 1 (HIBCRIT‐1) Study. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast‐enhanced magnetic resonance imaging (the High Breast Cancer Risk Italian 1 Study): final results. Invest Radiol 2011; 46: 94‐105.
- 8. Uematsu T, Yuen S, Kasami M, Uchida Y. Comparison of magnetic resonance imaging, multidetector row computed tomography, ultrasonography, and mammography for tumor extension of breast cancer. Breast Cancer Res Treat 2008; 112: 461‐474.
- 9. Sardanelli F, Newstead GM, Putz B, et al. Gadobutrol‐enhanced magnetic resonance imaging of the breast in the preoperative setting: results of 2 prospective international multicenter phase III studies. Invest Radiol 2016; 51: 454‐461.
- 10. Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta‐analysis in detection of multifocal and multicentric cancer. J Clin Oncol 2008; 26: 3248‐3258.
- 11. Brennan ME, Houssami N, Lord S, et al. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: systematic review and meta‐analysis of incremental cancer detection and impact on surgical management. J Clin Oncol 2009; 27: 5640‐5649.
- 12. Balleyguier C, Dunant A, Ceugnart L, et al. Preoperative breast magnetic resonance imaging in women with local ductal carcinoma in situ to optimize surgical outcomes: results from the randomized phase III trial IRCIS. J Clin Oncol 2019; 37: 885‐892.
- 13. Gonzalez V, Sandelin K, Karlsson A, et al. Preoperative MRI of the breast (POMB) influences primary treatment in breast cancer: a prospective, randomized, multicenter study. World J Surg 2014; 38: 1685‐1693.
- 14. Peters NHGM, van Esser S, van den Bosch MAAJ, et al. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: the MONET randomised controlled trial. Eur J Cancer 2011; 47: 879‐886.
- 15. Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet 2010; 375: 563‐571.
- 16. Sardanelli F, Trimboli RM, Houssami N, et al. Magnetic resonance imaging before breast cancer surgery: results of an observational multicenter international prospective analysis (MIPA). Eur Radiol 2022; 32: 1611‐1623.
- 17. Houssami N, Turner RM, Morrow M. Meta‐analysis of pre‐operative magnetic resonance imaging (MRI) and surgical treatment for breast cancer. Breast Cancer Res Treat 2017; 165: 273‐283.
- 18. Houssami N, Turner R, Macaskill P, et al. An individual person data meta‐analysis of preoperative magnetic resonance imaging and breast cancer recurrence. J Clin Oncol 2014; 32: 392‐401.
- 19. Eisen A, Fletcher GG, Fienberg S, et al. Breast magnetic resonance imaging for preoperative evaluation of breast cancer: a systematic review and meta‐analysis. Can Assoc Radiol J 2024; 75: 118‐135
- 20. Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta‐analysis of surgical outcomes. Ann Surg 2013; 257: 249‐255.
- 21. Gupta D, Billadello L. Breast MR imaging in newly diagnosed breast cancer. Radiol Clin North Am 2017; 55: 541‐552.
- 22. Ray KM, Hayward JH, Joe BN. Role of MR imaging for the locoregional staging of breast cancer. Magn Reson Imaging Clin N Am 2018; 26: 191‐205.
- 23. Australian Government Department of Health. New Medicare Benefit Schedule items for magnetic resonance imaging for breast cancer factsheet. Canberra: Australian Government, 2019. https://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Factsheet‐MRIBreastCa (viewed June 2025).
- 24. von Elm E, Altman DG, Egger M; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344‐349.
- 25. Australian Bureau of Statistics. Socio‐Economic Indexes for Areas (SEIFA), Australia. Canberra: ABS, 2023. https://www.abs.gov.au/statistics/people/people‐and‐communities/socio‐economic‐indexes‐areas‐seifa‐australia/latest‐release (viewed June 2025).
- 26. Staub LP, Lord SJ, Simes RJ, et al. Using patient management as a surrogate for patient health outcomes in diagnostic test evaluation. BMC Med Res Methodol 2012; 12: 12.
- 27. Guyatt GH, Tugwell PX, Feeny DH, et al. The role of before–after studies of therapeutic impact in the evaluation of diagnostic technologies. J Chron Dis 1986; 39: 295‐304.
- 28. Sardanelli F, Giuseppetti GM, Panizza P, et al. Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in fatty and dense breasts using the whole‐breast pathologic examination as a gold standard. Am J Roentgenol 2004; 183: 1149‐1157.
- 29. Checka CM, Chun JE, Schnabel FR, et al. The relationship of mammographic density and age: implications for breast cancer screening. Am J Roentgenol 2012; 198: W292‐W295.
- 30. MBS Online. Medicare Benefits Schedule – Item 63533. https://www9.health.gov.au/mbs/fullDisplay.cfm?type=item&q=63533&qt=item (viewed Aug 2025).
- 31. Mann GB, Skandarajah AR, Zdenkowski N, et al. Postoperative radiotherapy omission in selected patients with early breast cancer following preoperative breast MRI (PROSPECT): primary results of a prospective two‐arm study. Lancet 2024; 403: 261‐270.






Abstract
Objectives: To understand whether and how breast magnetic resonance imaging (MRI) at cancer diagnosis influences treatment planning, and whether subpopulations of patients with newly diagnosed breast cancer benefit in terms of most appropriate management.
Design: Multicentre prospective observational study.
Setting: Seven centres across New South Wales, Victoria and Western Australia during the period 15 September 2020 to 14 July 2022.
Participants: Patients with newly diagnosed early breast cancer meeting predefined criteria for whom multidisciplinary team normal practice deemed MRI would aid treatment planning.
Intervention: Preoperative contrast‐enhanced MRI.
Main outcome measures: Reasons for requesting MRI; pre‐MRI versus post‐MRI changes in treatment plans; changes justified by pathology findings.
Results: 387 eligible participants were enrolled. MRI was most frequently requested for dense breasts (252 [65%]), clinical and/or radiological size discrepancy (161 [42%]), multifocality (108 [28%]) and young age (105 [27%]). Change in treatment plan after MRI occurred for 198 participants (51% [95% CI, 46–56%]), including a change in breast surgery plan for 119 participants (31% [95% CI, 26–36%]). More mastectomies were planned after MRI (15%v 28%; absolute risk difference [RD], 13 percentage points [95% CI, 9–17]; P < 0.001), including unilateral mastectomy (14% v 24%; RD, 10 percentage points [95% CI, 6–14]; P < 0.001) and bilateral mastectomy (1% v 4%; RD, 3 percentage points [95% CI, 1–5]; P < 0.001). No increases in planned mastectomies occurred for women aged ≥ 70 years (RD, –3 percentage points [95% CI, –15 to 9]; or in those for whom neoadjuvant therapy was planned (RD, 2 percentage points [95% CI, –11 to 14]). Change in surgery was deemed justified by pathology findings in 75 of 88 women who experienced a change (85% [95% CI, 75–91%]).
Conclusions: Preoperative MRI findings led to changes in surgical management for a third of selected women with early breast cancer, increasing the mastectomy rate. In most cases, the changes were deemed appropriate. MRI findings did not change planned mastectomy in those aged ≥ 70 years, indicating that these women may not experience changes in surgical plans after such testing.