Asthma occurs when the normal development or function of the respiratory and immune systems are disturbed by environmental exposures that interact with genetic or biological predispositions. The developmental and early life origins of asthma are increasingly recognised, from the prenatal period through to critical windows in childhood. However, the asthma journey is not the same for all people, and a diagnosis can be made at any age; about 50% of cases are classified as early‐onset asthma, 50% as late‐onset asthma.1 A person diagnosed with asthma during childhood may experience symptoms throughout their life, or may have none during adulthood; yet others might have no symptoms during childhood but are diagnosed with asthma as adults. It is unclear whether asthma can actually turn on and off throughout life, or whether it persists in asymptomatic form. But what determines when symptomatic asthma appears? The development and presentation of asthma is complex, involving the interplay of potentially harmful exposures and underlying disease susceptibility. The CURE Asthma initiative was launched in Australia in 2024 as a collaborative, scientific, and systematic approach to finding a cure for asthma.2 In searching for a CURE, it is essential to consider the factors that determine who develops asthma, and why.
Exposures and insults: a matter of timing?
The timing and frequency of exposures and insults play critical roles in asthma development. Numerous risk factors during the different life stages have been identified (Box 1). Many adults with asthma report that their symptoms first appeared during infancy or early childhood, suggesting it is a crucial developmental window. As both the respiratory and immune systems mature during the first years of life, it is unsurprising that exposures and insults during fetal development and early life can lead to asthma development. Viral infections appear to play a key role during this critical period, particularly infections of the lower airway and those associated with wheeze or fever.3,4,5 People with early signs of atopy (such as eczema) as well as pre‐school wheeze are at particularly high risk of asthma. Environmental exposure at any point in life can be beneficial or detrimental, and it is important to consider an individual’s exposome; that is, the number and types of exposures during a life period (prenatal to adulthood). Detrimental environmental exposures can increase asthma risk for children (Box 2). The effects of such exposures can be exacerbated by poverty, poor nutrition or obesity, and lower socio‐economic status, poor quality housing, maternal stress, and adverse life events. For example, overcrowding is more frequent in low income households and facilitates transmission of infections, which in turn can increase the risk of asthma.
Individual exposures can have additive effects if experienced repeatedly or in succession, and can further disrupt underlying biological responses (Box 3). Reducing exposure to known risk factors throughout life is therefore one component of asthma management. Increasing protection against viral infections through vaccination, preventing allergic sensitisation, and public health campaigns to reduce smoking can reduce the prevalence of asthma symptoms.6,7 However, it is unrealistic to expect complete avoidance of all exposures; further, not all exposures during early life are detrimental. Children who spend their early years in farm environments, especially during their fetal development, are probably exposed to a greater diversity of microbes than other children, but their lifetime risk of asthma is lower.8
Further, there is no clear evidence that exposure to any single insult definitively results in asthma. Most children experience viral respiratory infections; young children have a mean of six to twelve viral infections each year.9,10 Most children experience mild symptoms, such as fever or cough; some have severe symptoms, including wheezing or breathing difficulties, but few children who experience viral‐induced wheeze later develop asthma. Similarly, not every adult exposed to fine particles at work or elsewhere will develop asthma. Whether an exposure causes harm and leads to asthma depends on the complex interplay of personal and environmental factors, including the timing of exposure, individual susceptibility, and the biological responses. This leaves the question: how does exposure lead to disease?
Asthma susceptibility and key drivers: what lies beneath the surface?
The multiple hit hypothesis of asthma posits that it develops when a person with an underlying susceptibility is subjected to repeated insults or exposures. Underlying biological changes, both systemic and specifically in the lung, can alter the way in which the body encounters and responds to the surrounding environment, setting the foundation for asthma development. Several key mechanisms are associated with asthma.
Genetics and individual susceptibility
A genetic component of asthma has long been recognised; children are more likely to develop asthma if one of their parents has asthma.11 Genome‐wide association studies have identified several genes associated with increased risk of asthma development.12 However, the findings of twin cohort studies indicate that asthma is not a purely genetic disease, as asthma can develop in just one of two identical twins.13 Individual susceptibility to exposures has not been well characterised by genome‐wide association studies, and may be better reflected by examining mechanistic pathways and how gene variations affect these pathways. Epigenetic modifications or epigenetic reprogramming, which alter gene expression without altering the genes themselves, are caused by changes in the environment or behaviour, and their important role in the pathogenesis of asthma is increasingly recognised.14 These modifications, which can be inherited, modify gene expression and orchestrate downstream processes, such as immune and epithelial cell responses. The plasticity and potential reversibility of the epigenome not only provide a potential target for interventions, but the finding that epigenetic changes are strongly linked with early life events could explain why the timing of exposures is critical.15
Immune responses that predispose people to allergy during development
Type 2‐biased immune responses16 — that is, immune responses primarily involving T‐helper cell type 2 (Th2) and type 2 innate lymphoid cells (ILC2) — are dominant during fetal development and early life. Exposures and insults after birth can either dampen (eg, microbes) or augment these responses (eg, allergens). Delayed immune maturation during early life increases the risk of more severe lower respiratory viral infections that can prolong the type 2 bias of the infant immune system, which can increase the risk of allergic sensitisation during early life.17,18
IgE, atopy, and increased asthma risk
Atopy, the predisposition to exaggerated IgE responses, is associated with greater risk of asthma. People with atopy who are exposed to environmental insults, such as allergens, viruses, bacteria, and pollution, produce unusually high levels of specific IgE antibodies to these otherwise innocuous stimuli. Antigen‐specific IgE responses induce mast cell degranulation, high‐affinity IgE receptor (FCeR1) expression on mast cells, eosinophils, and basophils, polyclonal B and T cell activation, NK cell activation, and oxidative stress, each of which is implicated in the pathogenesis of asthma.17,19
Immune responses in asthma
Clinical, epidemiological, and experimental evidence indicates that specific exposures and insults can alter immune responses to promote asthma development, and altered immune responses can increase disease severity in people with established asthma.20,21 Asthma can be subtyped according to the inflammatory cell count in induced sputum, reflecting their numbers in the airways (eosinophilic, non‐eosinophilic, neutrophilic, paucigranulocytic asthma), and these subtypes can be underpinned by a spectrum of both innate and adaptive (including those involving Th2, Th1, and Th17) immune responses.17
Immune responses in the lung microenvironment are important for the coordination, activation, and chemoattraction of immune cells from systemic sites, including the bone marrow. Recent studies have found that systemic immune responses, such as those detected in the blood, parallel local lung immune responses in people with asthma,22 indicating that these responses should be considered potentially modifiable targets for interventions.
Oxidative stress
Oxidative stress, the local imbalance between oxidants (such as reactive oxygen species: hydrogen peroxide, superoxide anion, hydroxyl radical) and antioxidants, is a key feature of asthma and is associated with increased inflammation (type 1 and type 2 immune responses and elevated proinflammatory cytokine levels) and greater disease severity. In asthma, mutations in genes associated with oxidative stress (eg, glutathione S‐transferase genes GSTM1, GSTP1, GSTT1) have been identified in genome‐wide association studies;11 further, many relevant exposures and insults (Box 2) cause oxidative stress.11 However, the degree to which asthma is explained by individual susceptibility to oxidative stress is unknown.
Altered lung structure
The airway epithelium is the first point of contact for inhaled substances and is an important modulator of the immune system. The airway epithelium is abnormal in asthma, with reported changes including reduced barrier integrity, delayed repair, goblet cell hyperplasia, and altered production of inflammatory mediators.23 Such defects can increase the vulnerability to external insults and exposures. Additionally, the epithelium of people with asthma does not respond to viruses in the same manner as do healthy epithelial cells,24,25 and exposures can therefore further damage the epithelium. A vicious cycle of injury and altered repair and responses can ensue, ultimately leading to epithelial remodelling.
The airway smooth muscle is fundamentally different in people with asthma and can contribute to airway narrowing, remodelling, and inflammation. These changes can include hypertrophy or hyperplasia (remodelling); changes in its intrinsic, structural, or mechanical properties; and altered interactions with the surrounding lung structure.26 While the basic mechanisms underlying airway smooth muscle changes in asthma are known, when they develop during life and whether they are contributors to the pathogenesis of asthma or consequences of asthma is less well established.
The microbiome
Microbiome diversity is reduced in people with asthma, and their bacterial load increased.27 Microbial diversity in early life is of fundamental importance for the maturation of the immune system, and dysbiosis can tip the balance between the innate and adaptive immune responses, leading to sensitisation and inflammation. Dysbiosis can promote environments that facilitate the migration of infectious pathogens from the upper to lower airway, increasing the risk of asthma.28 The maternal microbiome is also important, as it plays a major role in shaping the developing immune system and microbiome of the infant. Exposures experienced by the mother during pregnancy, including to antibiotics, can induce inflammation and oxidative stress, leading to altered immune priming of the fetus.29 The transmission of vaginal or gut microbes from the mother to the baby during birth also plays a critical role in the maturation of the baby’s immune system.
Asthma in First Nations Australians
It is unclear whether repeated exposures modify biological factors in people who develop asthma, or biological factors increase the risk of repeated exposures. A combination of functional deficits and ongoing exposures probably leads to a self‐perpetuating cycle that leads to asthma. Further research is needed to determine how these factors interact to cause disease, and whether exposures and biological factors differ between certain groups of people.
Asthma in First Nations Australians, in particular, requires more investigation. Asthma‐related mortality among First Nations Australians is almost double that for other Australians (2.5 v 1.3 deaths per 100 000 people).30 The prevalence of asthma among First Nations Australians is slightly higher than for other Australians, but varies from 6.1% in Central Australia to 31.2% in Victoria.31 Its prevalence increases with age, from 12% in children to 26% of people over 55 years of age.30 The risk factors are similar to those for all Australians, but rates of smoking, obesity, and physical inactivity are higher among First Nations Australians.30 The role played by atopy is unclear, especially in very remote communities, and very little is known about other biological factors. Determining the major risk factors and their mechanisms should be a priority given the high mortality and burden of disease associated with asthma in First Nations Australians.
Using our knowledge of exposures and mechanisms to find a CURE
Many questions about the development of asthma remain (Box 4). We still do not know the mechanisms by which prenatal and early life exposures affect immune system development, including the role of the maternal microbiome, and whether it is possible to prevent or reverse asthma development in susceptible people. Although numerous risk factors have been identified, we still do not know which combination or timing of exposures poses the greatest risk for people susceptible to asthma. The complex interplay between exposures, insults, and biological factors makes it difficult to identify specific mechanisms that cause asthma, but it also suggests several possibilities for intervention and cure, including:
- Early life: many children have recurring wheeze in early life, about 30% of whom develop persistent asthma. Intervention during early life could prevent permanent structural remodelling and improve long term lung function development. Early identification of allergic triggers and immunomodulatory strategies that advance immune maturation could be particularly helpful, and successful immunomodulation would have lifelong benefits.
- Childhood and adolescence: asthma during childhood is most closely linked with allergy and type 2 inflammation. The optimal use of conventional asthma medications, together with strategies to limit inflammation caused by allergen and environmental exposures, could be helpful.
- Adulthood: many adults have mild asthma that could be controlled by optimal use of conventional asthma medications. Whether there is a role for immunomodulatory agents and strategies that limit inflammation caused by allergen and environmental exposures is unknown.
Conclusions
The concept of combination therapy for treating cancer has taken centre stage as the most effective approach. Treatments work in a synergistic manner to optimise efficacy, effectively targeting the disease from a variety of directions. Many asthma medications are already combination therapies, but they require continued use to maintain their benefits and do not cure the disorder. A combine‐to‐cure approach will be essential for curing asthma, simultaneously preventing harmful exposures and directed at the underlying biological mechanisms. Responding to the problem from multiple angles, using new approaches (such as antioxidant therapy to reduce oxidative stress and selective immunomodulation to prevent infection), in addition to personalised treatment regimens, will be central to moving forward. A successful CURE will hinge on our ability to define and describe distinct interactions between exposures and biological mechanisms, and how these interactions contribute to asthma. In order to reverse the damage, we must first learn how and why the damage occurs. Building on these relationships and the interplay between biology and environment will provide the platform needed to pursue curative treatments.
Box 1 – Key insults and exposures associated with higher risk of asthma throughout life
|
Life stage |
Exposures and insults linked to asthma |
||||||||||||||
|
|
|||||||||||||||
|
Prenatal and perinatal periods |
|
||||||||||||||
|
|
|
||||||||||||||
|
|
|
||||||||||||||
|
|
|
||||||||||||||
|
Early life and childhood |
|
||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
Adulthood |
|
||||||||||||||
|
|
|
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 2 – Environmental exposures that can contribute to the exposome for an individual and are linked with asthma development
|
Type |
Source |
||||||||||||||
|
|
|||||||||||||||
|
Air pollution |
|
||||||||||||||
|
|
|
||||||||||||||
|
|
|
||||||||||||||
|
|
|
||||||||||||||
|
|
|
||||||||||||||
|
|
|
||||||||||||||
|
Chemicals |
|
||||||||||||||
|
|
|
||||||||||||||
|
|
|
||||||||||||||
|
|
|
||||||||||||||
|
Allergens |
|
||||||||||||||
|
|
|
||||||||||||||
|
|
|
||||||||||||||
|
|
|
||||||||||||||
|
Infections |
|
||||||||||||||
|
|
|
||||||||||||||
|
Workplace exposures |
|
||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 3 – The exposures and insults, and biological responses involved in the development of asthma. The timing and combination of exposures at different life stages (represented by lightning bolts) play major roles in its development
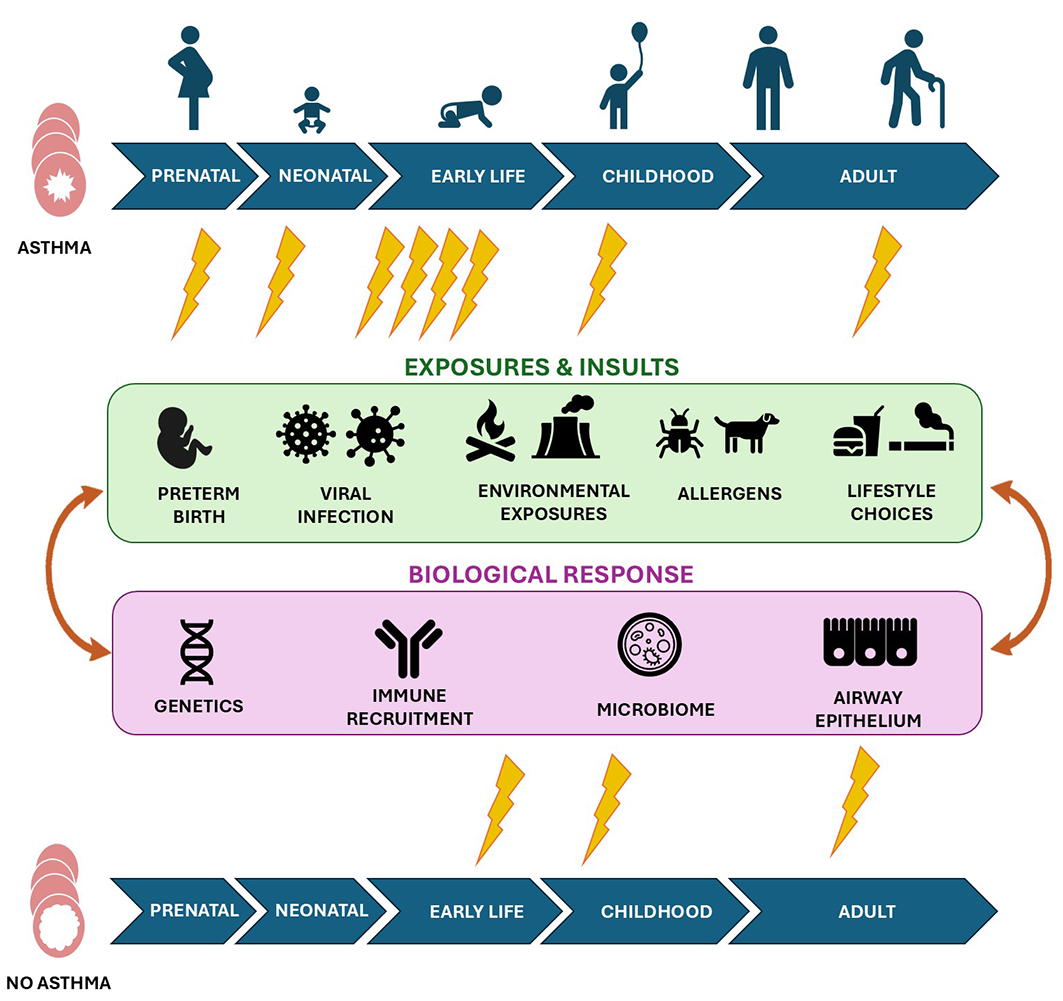
Box 4 – Key scientific questions that remain about the development and treatment of asthma
- Which combination of prenatal environmental exposures increases asthma risk?
- Will reducing the impact of childhood viral infections on respiratory and immune systems reduce asthma risk?
- Can we reset aberrant responses in the innate and adaptive immune systems in the lung during childhood to cure asthma?
- How much is asthma explained by individual susceptibility to systemic inflammation and oxidative stress?
- Can asthma be cured by using antioxidants to reduce oxidative stress and systemic inflammation?
Provenance: Not commissioned; externally peer reviewed.
- 1. Tan DJ, Lodge CJ, Walters EH, et al. Longitudinal asthma phenotypes from childhood to middle‐age: a population‐based cohort study. Am J Respir Crit Care Med 2023; 208: 132‐141.
- 2. Flynn A, Edmondson W, James A, et al. CURE Asthma: a unique opportunity for Australia. Med J Aust 2025; 223 (10 Suppl): 3‐8.
- 3. Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high‐risk children. Am J Respir Crit Care Med 2008; 178: 667‐672.
- 4. Kusel MM, Kebadze T, Johnston SL, et al. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur Respir J 2012; 39: 876‐882.
- 5. van Meel ER, Mensink‐Bout SM, den Dekker HT, et al. Early‐life respiratory tract infections and the risk of school‐age lower lung function and asthma: a meta‐analysis of 150 000 European children. Eur Respir J 2022; 60: 2102395.
- 6. Ciaccio CE, Gurley‐Calvez T, Shireman TI. Indoor tobacco legislation is associated with fewer emergency department visits for asthma exacerbation in children. Ann Allergy Asthma Immunol 2016; 117: 641‐645.
- 7. Davis MM, Halasyamani LK. COVID‐19 Vaccination and parent‐reported symptomatic child asthma prevalence. JAMA Netw Open 2024; 7: e2419979.
- 8. Campbell B, Raherison C, Lodge CJ, et al. The effects of growing up on a farm on adult lung function and allergic phenotypes: an international population‐based study. Thorax 2017; 72: 236‐244.
- 9. Teoh Z, Conrey S, McNeal M, et al. Burden of respiratory viruses in children less than 2 years old in a community‐based longitudinal US birth cohort. Clin Infect Dis 2023; 77: 901‐909.
- 10. Klee B, Diexer S, Langer S, et al. Acute respiratory tract infections during the first 6 years of life: results from the German birth cohort study LoewenKIDS. Int J Infect Dis 2025; 153: 107802.
- 11. Litonjua AA, Carey VJ, Burge HA, et al. Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am J Respir Crit Care Med 1998; 158: 176‐181.
- 12. Stikker BS, Hendriks RW, Stadhouders R. Decoding the genetic and epigenetic basis of asthma. Allergy 2023; 78: 940‐956.
- 13. Skadhauge LR, Christensen K, Kyvik KO, Sigsgaard T. Genetic and environmental influence on asthma: a population‐based study of 11 688 Danish twin pairs. Eur Respir J 1999; 13: 8‐14.
- 14. Ntontsi P, Photiades A, Zervas E, et al. Genetics and epigenetics in asthma. Int J Mol Sci 2021; 22: 2412.
- 15. Li S, Ye Z, Mather KA, et al. Early life affects late‐life health through determining DNA methylation across the lifespan: a twin study. eBioMedicine. 2022; 77: 103927.
- 16. Abu‐Raya B, Michalski C, Sadarangani M, Lavoie PM. Maternal immunological adaptation during normal pregnancy. Front Immunol 2020; 11: 575197.
- 17. Lambrecht BN, Ahmed E, Hammad H. The immunology of asthma. Nat Immunol 2025; 26: 1233‐1245.
- 18. Medeleanu MV, Qian YC, Moraes TJ, Subbarao P. Early‐immune development in asthma: a review of the literature. Cell Immunol 2023; 393‐394: 104770.
- 19. Froidure A, Mouthuy J, Durham SR, et al. Asthma phenotypes and IgE responses. Eur Respir J 2016; 47: 304‐319.
- 20. Camiolo MJ, Kale SL, Oriss TB, et al. Immune responses and exacerbations in severe asthma. Curr Opin Immunol 2021; 72: 34‐42.
- 21. Holgate ST. Innate and adaptive immune responses in asthma. Nat Med 2012; 18: 673‐683.
- 22. Horvat JC, Kim RY, Weaver N, et al. Characterization and inhibition of inflammasome responses in severe and non‐severe asthma. Respir Res 2023; 24: 303.
- 23. Heijink IH, Kuchibhotla VNS, Roffel MP, et al. Epithelial cell dysfunction, a major driver of asthma development. Allergy 2020; 75: 1902‐1917.
- 24. Frey A, Lunding LP, Ehlers JC, et al. More than just a barrier: the immune functions of the airway epithelium in asthma pathogenesis. Front Immunol. 2020; 11: 761.
- 25. Wark PAB, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005; 201: 937‐947.
- 26. An SS, Bai TR, Bates JHT, et al. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J 2007; 29: 834‐860.
- 27. Valverde‐Molina J, García‐Marcos L. Microbiome and asthma: microbial dysbiosis and the origins, phenotypes, persistence, and severity of asthma. Nutrients 2023; 15: 486.
- 28. Hufnagl K, Pali‐Schöll I, Roth‐Walter F, Jensen‐Jarolim E. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin Immunopathol 2020; 42: 75‐93.
- 29. Gao Y, Nanan R, Macia L, et al. The maternal gut microbiome during pregnancy and offspring allergy and asthma. J Allergy Clin Immunol 2021; 148: 669‐678.
- 30. Australian Institute of Health and Welfare. First Nations people with asthma. Updated 14 Dec 2023. https://www.aihw.gov.au/reports/chronic‐respiratory‐conditions/first‐nations‐people‐with‐asthma (viewed July 2025).
- 31. Howarth TP, Jersmann HPA, Majoni SW, et al. The “ABC” of respiratory disorders among adult Indigenous people: asthma, bronchiectasis and COPD among Aboriginal Australians: a systematic review. BMJ Open Respir Res 2023; 10: e001738.





None.
No relevant disclosures.
Author contribution:
Denby Evans: conceptualisation, writing (original draft), writing (review and editing). Peter D Sly: conceptualisation, writing (original draft), writing (review and editing). Paul Foster: conceptualisation, writing (review and editing). Chantal Donovan: conceptualisation, writing (original draft), writing (review and editing).