Asthma is an insidious disease that affects the lives of millions of people in Australia and hundreds of millions around the world.1 “Asthma” encompasses many different types of “asthmas”. Hope lies in recent findings regarding therapies that achieve clinical remission in people with specific types of asthma.2 Buoyed by these findings and advances in technology, we believe that scientific innovation holds the key to defining the asthmas, understanding their causes, and developing targeted treatments and ultimately cures. In this narrative review, we describe the key technological innovations that can translate these ambitions into clinical benefit.
Asthma encompasses several diseases with distinct presentations, currently categorised as six clinical groups: allergic asthma, non‐allergic asthma, adult‐onset asthma, cough‐predominant asthma, asthma with persistent airflow limitation, and asthma with obesity.3 However, disease characteristics differ even within these groups, and two major types have been defined, based on the form of airway inflammation: type 2‐high and type 2‐low asthma. This newer classification stems from the concept of asthma endotypes, in which classification is based on defined molecular pathways and, ideally, matched therapies.4 The challenge for discovery scientists is to further define and refine the asthma endotypes. Understanding these differences are important for developing precise treatments and cures.
Environmental and genetic factors contribute to the risk of asthma. Exposure to smoking, air pollutants, and certain infections increases the likelihood of developing asthma; sex, age, and ethnic background not only affect the risk but also influence the severity and type of asthma.5,6 The effectiveness of treatments depends on the asthma type; some can be easily managed with medications, others are more difficult to control. The symptoms of many children with early onset‐asthma become less severe with age. Further, type‐2 high asthma can largely be controlled and flare‐ups managed if patients comply with medication recommendations.7
Treatments for asthma include glucocorticoids, which have a broad effect on inflammation and immunity, usually combined with bronchodilators; they relieve symptoms, but they are not directed at the root cause of asthma.8 These medication types do not effectively control asthma in some people, and they must be taken as prescribed, the importance of which is seen during episodes of thunderstorm asthma. The combination of thunderstorms with high pollen levels can trigger sudden asthma flare‐ups in people with allergies or asthma; a very severe thunderstorm asthma event in Melbourne during the spring of 2016 led to high demand on emergency services and ten deaths in a single day.9 This tragic event, and smaller subsequent thunderstorm asthma events, underscore the need for better strategies to control and eventually cure asthma.
An estimated 5–10% of people with asthma have severe asthma, characterised by persistent symptoms despite treatment, or symptoms that can only be controlled by high medication doses.10 This debilitating condition greatly affects quality of life.11 While treatments for severe asthma have advanced markedly over the past two decades and therapies tailored to specific asthma characteristics are available, further improving the quality of life for people with severe asthma remains important.
Therapies that directly target the cause of asthma could fulfil this need, but they would require better understanding of its aetiology. Studying the cell populations involved in asthma is often difficult because of their paucity and the heterogeneity of patients in asthma cohort studies. Research methods and tools have rapidly improved over the past decade, including the capacity for simultaneous high‐dimensional analyses of more than forty parameters in single cells using spectral flow cytometry and powerful high‐throughput techniques that enable researchers to assess all genes (genomics), proteins (proteomics), metabolites (metabolomics), or microbes (metagenomics) (Box). In addition, recent breakthroughs in spatial transcriptomic and proteomic technology endow scientists with the ability to identify pathogenic cells in situ. These technological advances have opened new avenues of research, facilitating in depth investigations of difficult to study cell populations, leading to groundbreaking findings. With the development of more high‐throughput assays with greater sensitivity, sophisticated sequencing technology, and advanced computational tools, particularly the predictive ability of artificial intelligence and machine learning, we are poised for major breakthroughs in the study of asthma, supported by collaborations across diverse scientific fields.
In this article, we provide a detailed appraisal of the technologies that will be central to paving the pathway to a CURE for asthma. We focus on technologies most useful for discovering new disease mechanisms. We do not discuss important advances in non‐invasive sampling (eg, breathomics, particles in exhaled air [PExA]) or advances in imaging, from the whole organism level to lung tissue ultrastructure and cryo‐electron microscopy. We identified articles by searching PubMed and Google Scholar for “asthma”, “severe asthma”, “spatial technology”, “AI”, and “multi‐omics”, and reviewed reference lists in retrieved articles for further relevant publications. We applied no language or date range restrictions; when several sources covered the same technology, we gave priority to the most recent articles. We preferred original studies for specific methodologies.
Spectral flow cytometry
Immunophenotyping, or characterising the immune system of an individual by assessing cell markers, is critical in conditions with substantial variability. Profiling the immune system using flow cytometry can help characterise the disease endotype, but immunophenotyping methods have typically been low throughput, assessing only six to ten cell markers at a time. As most cell types require multiple markers for accurate identification, conventional immunophenotyping methods were limited and required a large amount of biological material for comprehensive immune system characterisation. The recent development of spectral flow cytometry has removed this barrier by increasing the assessment capacity to more than 40 markers per run using less than 2 mL blood.12 Further, the rigorous standardisation of spectral flow cytometers, such as the Cytek Aurora (https://cytekbio.com/pages/aurora), reduces variability between runs and machines, increasing the reproducibility and clearness of results. The immune system can now be characterised for large cohorts of people, facilitating definition of their asthma phenotype, as described in a recent study that used high‐dimensional profiling and other omics to go beyond characterisation of asthma as type 2‐high or type 2‐low.13 This approach will undoubtably improve treatment options.
Proteomics and metabolomics
Proteomics and metabolomics are often combined to provide a comprehensive overview of cellular processes. Both approaches are typically performed using mass spectrometry for the large scale profiling of small molecules and proteins in cells and tissues. Recent studies have used serum and sputum proteomics to classify asthma types14 and identify potential biomarkers in exhaled breath condensates.15
Metabolomics is the study of the complete set of small molecules known as metabolites (sugars, amino acids, lipids). Metabolomics provides a promising tool for investigating asthma pathogenesis and identifying biomarkers.16 For example, a recent study found that plasma metabolomics further clarified asthma subtypes in children.17 Measuring volatile organic compounds in exhaled breath could also be a non‐invasive diagnostic test,18 and analysis of exhaled breath led to the identification of a distinct allergic asthma signature in children.19 Breathomics has also been useful for therapeutic drug monitoring.20 Smaller amounts of patient material are needed as proteomics and metabolomics technology improves, using devices such as the Orbitrap Astral mass spectrometer (ThermoFisher).21
Single‐cell next‐generation sequencing
The rapid development of single cell sequencing technologies, followed by next‐generation sequencing assays such as cellular indexing of transcriptomes and epitope sequencing (CITE‐Seq), single cell RNA sequencing (scRNA‐Seq), and single cell transposase‐accessible chromatin sequencing (scATAC‐Seq),22 have provided novel insights into the heterogeneity of individual cell populations. Before these technologies were available, bulk cell populations were assessed together, limiting investigation to the overall picture of predefined populations and precluding insights into novel cell types or rare traits. Exploring heterogeneity in asthma is important for understanding the spectrum of disease presentation and severity, as well as its aetiology. For example, the most frequent form of asthma, type 2‐high asthma, is strongly linked with allergies. Memory B cells, a type of immune cell important for antibody production, are important in allergic responses, but people without allergies also have memory B cells, indicating heterogeneity within this cell population. Recent studies using scRNA‐Seq have implicated a subset of memory B cells in allergic conditions, MBC2 cells,23 suggesting a promising avenue of research for allergic asthma. With the advent and improvement of single cell sequencing technologies, the specific cells that contribute to asthma onset and pathogenesis can be identified and characterised, as recently reported for a human model of asthma exacerbation.24 Another study has illustrated how scATAC‐Seq can be used for the functional assessment and prioritisation of asthma risk loci.25 This technology has also been instrumental for constructing the human lung atlas (and its mouse counterpart), which has led to the identification of new lung cell types, such as the ionocyte,26 providing insights into how disease changes cell differentiation in the epithelium and the discovery of GAIN, a unique mucous gland‐associated immune niche for IgA‐secreting B plasma cells important for mucosal host defence.27,28 These technologies will not only improve disease endotyping, but also help identify new pathways for targeted therapies.
Spatial transcriptomics and proteomics
Individual cell populations are often investigated, but asthma is the manifestation of interactions between different cell types. For example, when a person with asthma encounters a trigger such as grass pollen, immune cells, including dendritic cells, lymphocytes, and eosinophils, are activated and interact, leading to cellular infiltration of the airway walls. This inflammation reduces the width of the airways, causing symptoms such as breathing difficulty and coughing. The interplay of the various cell types in the airways, and their location, determines the asthmatic response, and studying specific cell populations in isolation and without spatial context does not take tissue architecture and local features into account; cell niches and cell–cell communication within tissues contribute to both health and disease.
Spatial profiling, including spatial transcriptomics, proteomics, and metabolomics, can overcome this problem. Spatial techniques assess the expression or abundance of a target in intact tissue sections, facilitating assessment of the target of interest and the location of the target in the tissue.29 These technologies use either next‐generation sequencing or direct imaging.30 For transcriptomics and proteomics, higher multiplex spatial technologies enable the assessment of as many as 6000 molecules with sub‐cellular resolution, or the whole transcriptome at the cell type level, providing whole transcriptome profiling at subcellular resolution for research and drug development.
The upper airways are a highly organised system; the epithelial and immune cells are spatially and temporally organised to maintain tissue homeostasis by protecting against airborne infections.31 For example, different cells will be present in the airways depending on how much time has passed since an infection, so the cell compartment is also organised by time. Spatial technologies overcome the limitations of bulk and single‐cell sequencing associated with the dissociation and purification of cells that not only results in the loss of spatial information, but also the loss of rare cell types and cell types sensitive to removal from their supportive matrix.30 This could be particularly important in certain types of asthma, particularly allergic asthma, a type 2 inflammatory disease; eosinophils and mast cells have been difficult to process for scRNA sequencing.31 Several spatial technologies are now available, but a recent review of platforms discussed several unique features of GeoMx DSP (NanoString) for skin disease studies,32 including multimodal detection of proteins and RNA, the ability to segment by cell type, and no need for special histology slides or histology tissue processing instruments. Spatial transcriptomics and proteomics could revolutionise the understanding of immune and epithelial dysregulation and provide insights into how cells communicate with each other to cause asthma. Recently, this method has provided insights about pathogenic niches in chronic rhinosinusitis with nasal polyps, frequent in asthma, lung fibrosis, and chronic obstructive pulmonary disease.33,34
Multi‐omic integration
Research into the cause of asthma has typically focused on DNA mutations,35 but recent technological advances in transcriptomics, epigenetics (the study of factors other than DNA sequence that influence gene expression), proteomics, and metabolomics have facilitated a more comprehensive approach. Each of the omics can detect the differential expression of certain molecule types that could be associated with disease, but integrated analysis of the data provided by multiple omics technologies can elucidate the complex network of mechanisms that contribute to disease.36 Simultaneous profiling using the various omics, multi‐omics, is now possible with low input (small number of cells) and at single cell resolution, providing more information about the origin, state, and fate of cells. This multifaceted approach increases the prospect of identifying regulators of immune cells and disease by providing a more comprehensive picture of the network of regulatory molecular mechanisms. Exciting advances that combine transcriptomics, epigenomics, and spatial information are becoming available,37 and will be powerful tools for examining tissues from people with asthma, providing insights into disease pathogenesis and facilitating precision medicine interventions.
Multi‐omic research approaches could provide insights that accelerate the development of novel asthma therapies, and even a cure. The development of agents for blocking anti‐interleukin 33 (anti‐IL‐33) or thymic stromal lymphopoietin (anti‐TSLP) are examples of the success of a multidisciplinary research approach to the complex mechanisms underlying asthma. Single nucleotide polymorphisms (mutations in single bases of DNA sequences) identified in the genes for IL‐33 and TSLP genes were found to be associated with asthma development.38 Subsequent research has characterised how the proteins coded by these genes contribute to asthma progression, using different approaches from multiple fields, including molecular biology, cellular biology, and structural biology. Both TSLP and IL‐33 act as alarm signals for a variety of cell types, inducing type 2 immune responses, leading to allergies and allergic asthma.39 Based on such findings, agents that block TSLP and IL‐33 activity have been developed, and more are being investigated.40 This approach was also important in the recent discovery of the mitochondrial 15‐lipoxgenase ferroptosis pathway in severe asthma following the identification of altered oxidative phosphorylation in some people with asthma by the Unbiased Markers for the Prediction of Respiratory Disease Outcomes (UBIOPRED) and Severe Asthma Research Program (SARP) studies, leading to promising new biologic agents.41
Computational capabilities
As the described technological advances have increased the complexity and volume of data produced by single investigations, bioinformatics and computational biology are now critical for biological research, because sophisticated computer programs are needed to appropriately organise and analyse the available data. This is reflected not only by the increasing number of types of program, but also by the growth of computational fields such as artificial intelligence (AI).
AI typically refers to the ability to mimic human thinking and decision making, including characteristics such as perceptiveness, logic, and learning from experience. As biological research gathers large amounts of data with the aim of detecting patterns in the data, AI is especially suited for bioinformatics and computational biology because of its capacity to extract information by rapidly processing and analysing large datasets in an automated fashion. AI can not only be applied to analysing new asthma‐related datasets, but also to mining and re‐analysing earlier asthma datasets to detect patterns that had previously been missed because of the limitations of available computational tools.
Generative AI technologies, such as ChatGPT42 and AlphaFold,43 have revolutionised text and image generation and protein structure prediction using synthetic data, powered by large, pre‐trained foundation models that could transform health care.44 Using large medical datasets, these models can be fine tuned to predict clinical outcomes and personalise treatment with limited additional data (few‐shot learning).45 Together with digital twins (virtual representations of patients), these innovations herald a new era in precision and personalised medicine.46
AI methods could revolutionise asthma care.47 Traditional discriminative machine learning models are typically used for classification tasks, such as predicting asthma subtypes, or for regression tasks, such as forecasting clinical variables. However, generative AI could generate synthetic data, effectively creating digital twins of patients.46 This approach could replace propensity score methods for defining control arms in clinical trials. Extending this concept by linking patients with their virtual representations could enable the simulation of events such as asthma exacerbations, and the identification of biomarkers that predict therapeutic response. This innovation opens the possibility of conducting virtual trials, a new frontier in precision medicine.
Virtual representations of cells could be used for the investigation of asthma aetiologies by using generative AI and digital twins44,46,48 to model tissue development at single cell and spatial resolution. Spatial resolution refers to being able to see the cells within the natural geography of the tissue; it can also be described as studying the cells within the context of the tissue structure. By analysing single cell, spatial, and bulk transcriptomics data and integrating molecular subtypes and genetic variants, digital twins can be used to follow cell development in health and disease, with a focus on asthma exacerbations. Velocity vectors from RNA‐splicing variants can be calculated for each cell in order to predict changes in gene expression and development.49 Using advanced AI techniques, including transformers and variational autoencoders,50,51 digital twins can be used to project cellular trajectories, simulating lung development in healthy people and people with asthma. Through better knowledge of tissue architecture development, these technologies could provide insights into disease pathologies, identify novel drug targets, and improve clinical outcomes.
Multi‐omic integration using AI is a powerful approach to unravelling the complex molecular mechanisms of asthma.52 By combining data from genomics, transcriptomics, proteomics, and epigenomics, AI methods could identify key biomarkers and molecular signatures associated with asthma subtypes, disease progression, and therapeutic responses.53 Advanced machine learning models, such as deep learning and generative AI, enable the integration of these diverse datasets, identifying patterns and interactions between genetic variants, gene expression, protein levels, and epigenetic modifications.46,54 This holistic approach not only enhances our understanding of asthma pathophysiology, but also facilitates the development of personalised treatment strategies, predicting outcomes more accurately and optimising clinical interventions. These techniques are even more powerful when guided by the combined expertise and disease insights of basic and clinical translational teams.
Barriers to be overcome
Implementing these new technologies in clinical settings is a major challenge, and many cannot yet be used on a large scale. Giving priority to those required for diagnosis and determining how they can be integrated with AI will be the keys to success. This will require funding for equipment, computing power, and experimentation. Further, developing expertise, attracting scientists to work in asthma research, and promoting collaboration between clinicians and scientists is needed. Reducing the mismatch between the burden of asthma and the funding of asthma research55 by providing support for long term scientist–clinician collaboration would support the vision of the CURE asthma initiative.
The use of digital twins in health care raises important ethical questions, including personal privacy, data governance, and the risk of outputs that could lead to unsafe or misleading clinical recommendations. Comprehensive user education and the involvement of clinicians throughout the decision‐making process will be needed.
Future directions
Almost 11% of Australians have asthma. However, hope for a cure is provided by the fact that clinical remission has been achieved for some people treated with specific biologic medications.56 Moreover, better understanding of the mechanisms underlying spontaneous asthma remission57 is now achievable. Over the coming decade, we envisage that implementing the technologies discussed in this article will be critical for clinical translation towards cure by:
- providing precision diagnoses for people with asthma, ensuring that they receive optimal treatments;
- integrating the predictive powers of AI to predict clinical outcomes, identify biomarkers, and design virtual clinical trials; and
- identify the causes of asthma in order to inform the discovery of novel disease‐modifying and curative therapies.
The use of these technologies will be essential for research into disease pathogenesis, and developing precision diagnoses and therapies that will undoubtably contribute to ending the burden of asthma.
Box – How the omics facilitate advances toward precision asthma diagnosis and treatment*
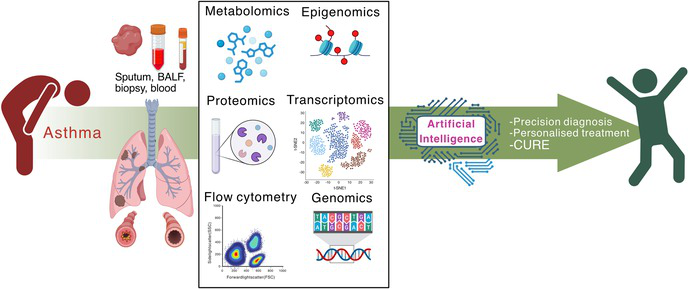
* Sputum, bronchoalveolar lavage fluid (BALF), lung biopsy, or blood samples collected from people with asthma can be analysed using a range of omics technologies to obtain diagnoses of asthma endotypes using multi‐omics integration. Data will also be provided to artificial intelligence systems that will enhance the predictive power for precision diagnoses and treatments, and inform clinical trials. The promise of these analyses is to develop precision diagnoses that facilitate personalised treatments and potentially cures for asthma. The image was prepared in BioRender (www.biorender.com).
Provenance: Not commissioned; externally peer reviewed.
- Sara Quon1
- Timothy M Johanson1
- Ridhima Wadhwa2
- Alen Faiz3
- Anthony Flynn4
- Gary P Anderson5
- Amanda J Cox6
- Nicholas P West6,7
- Michael P Menden5,8
- Rhys S Allan1
- 1 Walter and Eliza Hall Institute of Medical Research, Melbourne, VIC
- 2 Centenary Centre for Inflammation, Sydney, NSW
- 3 The University of Technology Sydney, Sydney, NSW
- 4 Asthma Australia Ltd, Sydney, NSW
- 5 Bio21 Molecular Science and Biotechnology Institute, the University of Melbourne, Melbourne, VIC
- 6 Griffith University, Gold Coast, QLD
- 7 Menzies Health Institute Queensland, Gold Coast, QLD
- 8 Institute of Computational Biology, Helmholtz Munich, Munich, Germany
We acknowledge funding support from the National Health and Medical Research Council, the Australian Research Council, the Stafford Fox Medical Research Foundation, the Veith Foundation, ENA Respiratory Pty Ltd, GSK, and Sanofi‐Regeneron.
Nicholas P West received research funding from ENA Respiratory Pty Ltd, GSK, and Sanofi‐Regeneron, and conference travel support from GSK. This funding was not related to the development or writing of this manuscript.
Authors’ contributions:
All authors: writing (original draft); writing (review and editing).
- 1. GBD 2021 Asthma and Allergic Diseases Collaborators. Global, regional, and national burden of asthma and atopic dermatitis, 1990–2021, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Respir Med 2025; 13: 425‐446.
- 2. McDowell PJ, McDowell R, Busby J, et al; UK Severe Asthma Registry. Clinical remission in severe asthma with biologic therapy: an analysis from the UK Severe Asthma Registry. Eur Respir J 2023; 62: 2300819.
- 3. Rajvanshi N, Kumar P, Goyal JP. Global Initiative for Asthma guidelines 2024: an update. Indian Pediatr 2024; 61: 781‐786.
- 4. Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008; 372: 1107‐1119.
- 5. Jayasooriya SM, Devereux G, Soriano JB, et al. Asthma: epidemiology, risk factors, and opportunities for prevention and treatment. Lancet Respir Med 2025; 13: 725‐738.
- 6. Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol 2020; 42: 5‐15.
- 7. Rehman N, Morais‐Almeida M, Wu AC. Asthma across childhood: improving adherence to asthma management from early childhood to adolescence. J Allergy Clin Immunol Pract 2020; 8: 1802‐1807.
- 8. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol 2011; 163: 29‐43.
- 9. Thien F, Beggs PJ, Csutoros D, et al. The Melbourne epidemic thunderstorm asthma event 2016: an investigation of environmental triggers, effect on health services, and patient risk factors. Lancet Planet Health 2018; 2: e255‐e263.
- 10. Hekking PPW, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol 2015; 135: 896‐902.
- 11. Foster JM, McDonald VM, Guo M, Reddel HK. “I have lost in every facet of my life”: the hidden burden of severe asthma. Eur Respir J 2017; 50: 170076.
- 12. Spasic M, Ogayo ER, Parsons AM, et al. Spectral flow cytometry methods and pipelines for comprehensive immunoprofiling of human peripheral blood and bone marrow. Cancer Res Commun 2024; 4: 895‐910.
- 13. Camiolo MJ, Zhou X, Oriss TB, et al. High‐dimensional profiling clusters asthma severity by lymphoid and non‐lymphoid status. Cell Rep 2021; 35: 108974.
- 14. Asamoah K, Chung KF, Zounemat Kermani N, et al; U‐BIOPRED Study Group. Proteomic signatures of eosinophilic and neutrophilic asthma from serum and sputum. EBioMedicine 2024; 99: 104936.
- 15. Hara R, Takeda Y, Enomoto T, et al. Potential asthma biomarkers identified by nontargeted proteomics of extracellular vesicles in exhaled breath condensate. J Allergy Clin Immunol Glob 2025; 4: 100432.
- 16. Xu S, Panettieri RA, Jude J. Metabolomics in asthma: a platform for discovery. Mol Aspects Med 2022; 85: 100990.
- 17. Mendez KM, Kachroo P, Prince N, et al. Exploring the varied clinical presentation of pediatric asthma through the metabolome. Am J Respir Crit Care Med 2025; 211: 737‐748.
- 18. Bos LD, Sterk PJ, Fowler SJ. Breathomics in the setting of asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol 2016; 138: 970‐976.
- 19. Houssni L, Srdjan M, Tobias B, et al. Breath profiles in paediatric allergic asthma by proton transfer reaction mass spectrometry. BMJ Open Respir Res 2025; 12: e003223.
- 20. Brinkman P, Ahmed WM, Gomez C, et al; U‐BIOPRED Study Group. Exhaled volatile organic compounds as markers for medication use in asthma. Eur Respir J 2020; 55: 1900544.
- 21. Stewart HI, Grinfeld D, Giannakopulos A, et al. Parallelized acquisition of orbitrap and astral analyzers enables high‐throughput quantitative analysis. Anal Chem 2023; 95: 15656‐15664.
- 22. Salma M, Andrieu‐Soler C, Deleuze V, Soler E. High‐throughput methods for the analysis of transcription factors and chromatin modifications: low input, single cell and spatial genomic technologies. Blood Cells Mol Dis 2023; 101: 102745.
- 23. Koenig JFE, Knudsen NPH, Phelps A, et al. Type 2‐polarized memory B cells hold allergen‐specific IgE memory. Sci Transl Med 2024; 16: eadi0944.
- 24. Alladina J, Smith NP, Kooistra T, et al. A human model of asthma exacerbation reveals transcriptional programs and cell circuits specific to allergic asthma. Sci Immunol 2023; 8: eabq6352.
- 25. Wei J, Resztak JA, Ranjbaran A, et al. Functional characterization of eQTLs and asthma risk loci with scATAC‐seq across immune cell types and contexts. Am J Hum Genet 2025; 112: 301‐317.
- 26. Plasschaert LW, Zilionis R, Choo‐Wing R, et al. A single‐cell atlas of the airway epithelium reveals the CFTR‐rich pulmonary ionocyte. Nature 2018; 560: 377‐381.
- 27. Madissoon E, Oliver AJ, Kleshchevnikov V, et al. A spatially resolved atlas of the human lung characterizes a gland‐associated immune niche. Nat Genet 2023; 55: 66‐77.
- 28. Schiller HB, Montoro DT, Simon LM, et al. The Human Lung Cell Atlas: a high‐resolution reference map of the human lung in health and disease. Am J Respir Cell Mol Biol 2019; 61: 31‐41.
- 29. Marx V. Method of the Year: spatially resolved transcriptomics. Nat Methods 2021; 18: 9‐14.
- 30. Krull D, Haynes P, Kesarwani A, et al. A best practices framework for spatial biology studies in drug discovery and development: enabling successful cohort studies using digital spatial profiling. J Histotechnol 2025; 48: 7‐26.
- 31. Wang W, Xu Y, Wang L, et al. Single‐cell profiling identifies mechanisms of inflammatory heterogeneity in chronic rhinosinusitis. Nat Immunol 2022; 23: 1484‐1494.
- 32. Cho C, Haddadi NS, Kidacki M, et al. Spatial transcriptomics in inflammatory skin diseases using GeoMx digital spatial profiling: a practical guide for applications in dermatology. JID Innov 2025; 5: 100317.
- 33. Mayr CH, Santacruz D, Jarosch S, et al. Spatial transcriptomic characterization of pathologic niches in IPF. Sci Adv 2024; 10: eadl5473.
- 34. Rojas‐Quintero J, Ochsner SA, New F, et al. Spatial transcriptomics resolve an emphysema‐specific lymphoid follicle b cell signature in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2024; 209: 48‐58.
- 35. Thomsen SF. Genetics of asthma: an introduction for the clinician. Eur Clin Respir J 2015; 2: https://doi.org/10.3402/ecrj.v2.24643.
- 36. Chen C, Wang J, Pan D, et al. Applications of multi‐omics analysis in human diseases. MedComm (2020) 2023; 4: e315.
- 37. Li H, Bao S, Farzad N, et al. Spatially resolved genome‐wide joint profiling of epigenome and transcriptome with spatial‐ATAC‐RNA‐seq and spatial‐CUT&Tag‐RNA‐seq. Nat Protoc 2025; 20: 2383‐2417.
- 38. Torgerson DG, Ampleford EJ, Chiu GY, et al. Meta‐analysis of genome‐wide association studies of asthma in ethnically diverse North American populations. Nat Genet 2011; 43: 887‐892.
- 39. Calderon AA, Dimond C, Choy DF, et al. Targeting interleukin‐33 and thymic stromal lymphopoietin pathways for novel pulmonary therapeutics in asthma and COPD. Eur Respir Rev 2023; 32: 220144.
- 40. Salvati L, Maggi L, Annunziato F, Cosmi L. Thymic stromal lymphopoietin and alarmins as possible therapeutical targets for asthma. Curr Opin Allergy Clin Immunol 2021; 21: 590‐596.
- 41. Yamada K, St Croix C, Stolz DB, et al. Compartmentalized mitochondrial ferroptosis converges with optineurin‐mediated mitophagy to impact airway epithelial cell phenotypes and asthma outcomes. Nat Commun 2024; 15: 5818.
- 42. Dave T, Athaluri SA, Singh S. ChatGPT in medicine: an overview of its applications, advantages, limitations, future prospects, and ethical considerations. Front Artif Intell 2023; 6: 1169595.
- 43. Abramson J, Adler J, Dunger J, et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024; 630: 493‐500.
- 44. Bordukova M, Makarov N, Rodriguez‐Esteban R, et al. Generative artificial intelligence empowers digital twins in drug discovery and clinical trials. Expert Opin Drug Discov 2024; 19: 33‐42.
- 45. Ma J, Fong SH, Luo Y, et al. Few‐shot learning creates predictive models of drug response that translate from high‐throughput screens to individual patients. Nat Cancer 2021; 2: 233‐244.
- 46. Bordukova M, Arneth AJ, Makarov N, et al. Generative AI and digital twins: shaping a paradigm shift from precision to truly personalized medicine. Expert Opin Drug Discov 2025; 20: 821‐826.
- 47. Exarchos KP, Beltsiou M, Votti CA, Kostikas K. Artificial intelligence techniques in asthma: a systematic review and critical appraisal of the existing literature. Eur Respir J 2020; 56: 2000521.
- 48. Makarov N, Bordukova M, Quengdaeng P, et al. Large language models forecast patient health trajectories enabling digital twins. NPJ Digit Med 2025; 8: 588.
- 49. Bergen V, Lange M, Peidli S, et al. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat Biotechnol 2020; 38: 1408‐1414.
- 50. Gayoso A, Weiler P, Lotfollahi M, et al. Deep generative modeling of transcriptional dynamics for RNA velocity analysis in single cells. Nat Methods 2024; 21: 50‐59.
- 51. Lange M, Bergen V, Klein M, et al. CellRank for directed single‐cell fate mapping. Nat Methods 2022; 19: 159‐170.
- 52. Wei K, Qian F, Li Y, et al. Integrating multi‐omics data of childhood asthma using a deep association model. Fundam Res 2024; 4: 738‐751.
- 53. Boniolo F, Dorigatti E, Ohnmacht AJ, et al. Artificial intelligence in early drug discovery enabling precision medicine. Expert Opin Drug Discov 2021; 16: 991‐1007.
- 54. He X, Liu X, Zuo F, et al. Artificial intelligence‐based multi‐omics analysis fuels cancer precision medicine. Semin Cancer Biol 2023; 88: 187‐200.
- 55. Williams S, Sheikh A, Campbell H, et al; Global Health Respiratory Network. Respiratory research funding is inadequate, inequitable, and a missed opportunity. Lancet Respir Med 2020; 8: e67‐e68.
- 56. Chipps BE, Lugogo N, Carr W, et al. On‐treatment clinical remission of severe asthma with real‐world longer‐term biologic use. J Allergy Clin Immunol Glob 2025; 4: 100365.
- 57. Thomas D, McDonald VM, Pavord ID, et al. Asthma remission: what is it and how can it be achieved? Eur Respir J 2022; 60: 2102583.





Summary