Asthma is a distressing … malady; the patient lives in dread of the attacks, which are relieved but not cured by treatment. William Osler, 18921
Can my asthma be cured? No, there is not yet a total cure for asthma. Global Initiative for Asthma, 20252
Is asthma fundamentally incurable? We would not accept this proposition for most serious diseases. Cancer researchers seek to cure cancer. Yet Osler’s dictum, penned more than a century ago, and clinical experience during the asthma crises of the 1960s, 1980s, and 1990s, during which the incidence and prevalence of asthma remained stubbornly high and its severity and mortality increased,3 have engendered this pessimism. Despite significant recent progress in its management, the widely held view is that asthma is a treatable but incurable disease.
We cannot accept this situation. The burden of asthma in Australia, among the highest in the world,4 is too great and the need for solutions too compelling. Patients, clinicians, and researchers want cures.5,6 They have been achieved for other diseases; why not asthma? The CURE Asthma initiative challenges the pessimism that it is incurable. Its goal is to eliminate asthma by discovering curative therapies. To do this we have declared our bold but realistic ambition to cure asthma and have established a national roadmap of translational research that will deliver new therapies that reverse the fundamental molecular causes of the different forms of asthma. We argue that research, industry, technology, innovation and government bodies in Australia be brought together and that the technical and financial resources needed for sustained discovery and translational research to find cures be provided.
The CURE Asthma initiative was developed by Asthma Australia as the result of its research prioritisation consultations with patients and their carers and academic, policy, and government bodies regarding the 2023 National Asthma Research Agenda.6 These consultations focused on important practical aspects of asthma management, including improving day‐to‐day care. They remain pressing needs, but a bolder challenge was advanced during the 2023 consultations: should curing asthma be a priority? It is remarkable that, when the possibility of finding cures was shared with the Asthma Australia Consumer Advisory Council, the delegates responded: “we were always told it is incurable”, and “why didn’t we do this 30 years ago?” People with asthma and their families want cures.
Just as the statement “we choose to go to the Moon” has become emblematic of world‐changing research, we propose that declaring the ambition to CURE asthma will open the imagination to transformative solutions. The purpose of this supplement is more than to provide a brief narrative of how and why the CURE Asthma Initiative was established. It is also more than to lionise the world‐leading position of Australia in advanced asthma research, built on decades of medical research support that has established exemplary longitudinal clinical cohorts, among the longest established and best characterised in the world, but a barely tapped resource. CURE Asthma is more than the outline of our research program, the scientific rationale and immense promise of which is explored by field experts in the articles comprising this supplement. The purpose is, like the United States National Cancer Act of 1971,7 a call to arms, to imagine a moonshot.
The size of the problem and the opportunity
We call for no more me‐too medicines but a commitment to develop treatment approaches that focus on prevention and cure. Lancet Commission, 2018.5
A cure for asthma would not only transform our daughter’s life but also our family’s. It would free my daughter from the constant battles she faces, not just with her health but also with missing out on school, activities and having a carefree childhood. Mother of “Oakley”, New South Wales, 2024.
Although many people believe the asthma problem is now under control,5 given the major improvements in care introduced from the 1990s to the 2010s,8,9 the burden of asthma remains unacceptably high, death rates are again rising (Box 1), and there are no disease‐modifying or curative therapies.5 Funding of basic research into the fundamental molecular mechanisms of asthma, especially of mild and moderate disease, is low given the burden of disease.11,12,13 Many people with asthma and their clinicians simply accept that they must live with their symptoms, exacerbations, and often permanent damage and lifetime sequelae.14 This cannot continue.
Despite widespread complacency, asthma remains a major health problem in Australia, affecting about 2.8 million people (11% of the population), one of the highest prevalence rates in the world (United States, United Kingdom: about 8%).15,16 Its prevalence is particularly high among children, First Nations people, and the socio‐economically disadvantaged.17 About 400 000 children have asthma, making it the leading cause of disease burden among those aged 0–14 years, and it is the cause of more hospitalisations of children than any other chronic condition.9,17,18 Children of pre‐school age with asthma are 87% more likely than other children to develop anxiety disorders in later childhood,19 and children hospitalised with asthma are more likely to fall behind academically.20
Further, asthma outcomes are strongly influenced by social determinants of health; its prevalence and disease burden are linearly correlated with social deprivation.17,21 Social determinants influence asthma both directly, by increasing disease susceptibility, and indirectly, through the hazards of exposures and insults, access to health care, nutrition, and lifestyle factors. Despite great resilience and the remarkable examples of Aboriginal community‐controlled progressive health care models, the difficulties experienced by Aboriginal and Torres Strait Islander people are particularly great22 (Box 2).
A 2016 online survey of 1333 adults with severe asthma and caregivers of children with severe persistent asthma from nine countries found that its impact on quality of life, asthma control, exacerbations, and psychological wellbeing had not changed since a similar 2004–05 survey, despite the introduction of targeted biological pharmaceuticals.30 Mild and moderate asthma also cause a substantial health burden for the general population. A 2021 Australian Bureau of Statistics survey found that people with asthma were less likely to report very good or excellent health.31 International surveys have also found that people with asthma are more likely to report psychological distress than respondents without asthma or with other health conditions.32 In 2023, asthma caused more than 30 000 hospitalisations in Australia,33 97 000 emergency department visits,34 and 474 deaths,10 contributing to an estimated economic cost exceeding $28 billion, $24 billion of which was attributed to the aggregate burden experienced by people with asthma.35 This estimate did not include the large impact on parents and carers, and the figures also excluded the substantial morbidity and costs associated with treatment side effects, particularly those of systemic corticosteroid therapy, including osteoporosis, metabolic and endocrine disorders, glaucoma, hypertension, and increased infection risk.36
These statistics only dimly reflect the challenges faced by clinicians. Childhood is often described as the silent years of asthma, as it is the period when diagnosis is difficult and objective tools for guiding treatment and assessing progress are few.37 It is striking and unacceptable that we still do not have molecular diagnostic methods. Clinicians must rely on often unreliable symptom reports by parents,38 as validated biomarkers and lung function tests for young children are limited. Further, childhood is a critical period of lung development. Even if diagnosis is optimised, no therapy modifies the disease course.5 Inhaled corticosteroids, the usual standard of care, suppress inflammation and are effective,39 but their typically intermittent use does not eliminate symptoms, completely prevent exacerbations in the long term, or alter the trajectory of the disease.5 Inhaled corticosteroids can be lifesaving but they are not curative.
In adulthood, by which time much of the lung damage caused by asthma has already occurred if its onset was during childhood, new problems arise. Adherence to medication is low and many people with persistent symptoms disengage from treatment.40 Despite new treatment paradigms, such as combinations of inhaled corticosteroids and long‐acting β‐adrenoceptor agonists that can be used as needed (anti‐inflammatory relievers), many people and clinicians continue to rely on short‐acting bronchodilators, and short‐course oral corticosteroids for flare‐ups. Between 2012 and 2021, nationally representative surveys in Australia found declining levels of self‐management by adults with asthma: fewer people regularly used inhaled corticosteroid preventers, poor symptom control was more frequent, and the number of self‐reported asthma‐related hospitalisations quadrupled.40 Current treatments can be adapted to individual patient needs, but often fail to meet them. The hospitalisation rate for Australians with asthma is higher than in other OECD countries,4 and excessive use of systemic corticosteroid therapy adds further harm.41 Previous advances in asthma management reduced asthma‐related mortality, but it is again rising,10 and we have not done anything to relieve the possibly rising burden of daily symptoms and exacerbations. We have failed to build on our successes to reduce the increasing day‐to‐day cost and burden of asthma.
Our mission to CURE asthma by attacking its root causes cannot distract from the continuing and pressing need to translate already available knowledge into better practice. The importance of using available treatments better now cannot be overstated. However, even were we to achieve 100% medication adherence, we estimate that direct asthma medication costs in Australia would exceed $1 billion each year and would still not eliminate the asthma burden. It is clear that a substantial shift in the quality and personalisation of care is urgently needed, but no current treatment is truly disease‐modifying or curative for most people with asthma, particularly those for whom social determinants of health play a major role. The CURE Asthma mission focuses on fundamentally attacking the underlying mechanisms of disease, and levelling the playing field for all, across all socio‐demographic domains.
The significant medical, social, and economic burden of asthma is a strategic opportunity for Australian biomedical research commercialisation, especially as funding agencies increasingly prioritise support for research with the potential for economic impact. The annual value of the global asthma therapeutics market is projected to reach nearly US$60 billion by 2030,42 providing a highly competitive environment in which curative therapies could redefine the standard of care. Precedents in other fields — such as triple‐modulator therapy for cystic fibrosis43 (US$36 billion by 203144), glucagon‐like peptide‐1 (GLP‐1) agonists for metabolic disease45 (US$71 billion by 203246), and immune checkpoint inhibitors for cancer47 (US$150 billion by 203148) indicate that transformative treatments can reshape markets and even influence national economic figures.49 These successes have generated intense commercial interest in curative asthma therapies and indicate the enormous opportunity for Australian biomedical research commercialisation.
Medical research has entered a period of immense opportunity in which fundamental research into disease mechanisms, linked with new methods of drug design and precision medicine development, are accelerating rapidly and synergistically, pushed forward by profound advances in machine learning and artificial intelligence.50 We are finally cutting through once inextricably tangled Gordian knots of pathobiology and discovering cures in other diseases. Most recently, transformative medicines for β‐thalassemia, adenosine deaminase deficiency, and aromatic l‐amino acid decarboxylase deficiency have been registered.51,52,53 Asthma is not as simple as rare monogenetic diseases. It is heterogenous, polygenetic, and comprises distinct endotypes, but these breakthrough strategies presage the future of medicine, in which we can build upon advances in molecular and computational science to accelerate progress towards cures.
A conceptual shift is already underway, led by advances in treating severe asthma with biological agents; treatment goals are moving from symptom control to sustained on‐treatment remission.54,55 This approach will be expanded to include people with mild and moderate disease. Conceptually, on‐treatment remission can be seen as a stepping stone to off‐treatment cures. The time to act is now.
Are cures scientifically feasible?
Incredible technological advances are enabling us to increase our understanding of disease biology at a molecular level and discover what is driving many of our most complex diseases. The more knowledge we gain, the better we can predict the clinical success of our candidate drug molecules, accelerating their delivery to get the right treatments to the right patients. Mene Pangelos, former executive vice‐president, AstraZeneca.56
Recent research findings suggest that curing asthma is scientifically feasible. As asthma comprises multiple distinct endotypes, “cure” will mean a family of distinct solutions rather than a single effective intervention. Long term findings of Australian and overseas cohort studies indicate that some people with asthma can experience spontaneous lasting remission.57 Asthma is an acquired condition, and complete spontaneous remission and on‐treatment remission57 can now be achieved in some patients, which also indicates that asthma can be cured. Defining the molecular basis for the clinical features of lasting off‐treatment remission is one of the keys to developing curative strategies.
In an Australian registry data‐based assessment of the effectiveness of biological therapies, 23–29% of adults with asthma achieved clinical remission (defined as no exacerbations and no oral corticosteroid use) and 19–25% achieved both clinical remission and stable lung function.58 Given that so many people receiving such therapies achieve on‐treatment remission, interest in identifying “super‐responders” (those for whom the therapy is profoundly effective) is growing, supporting proposals to define remission as a formal treatment goal.59 Further, a placebo‐controlled trial of another Australian‐led treatment innovation, macrolide therapy for severe asthma (the AMAZES trial), found that clinical remission was achieved in as many of 50% of intervention group participants with eosinophilic or non‐eosinophilic asthma.60 These findings have changed care for people with asthma around the world.61
While our goal of cures for asthma is ambitious, the scientific progress described in the articles in this supplement supports their feasibility. Genome‐wide association studies have identified genetic variants associated with complete remission, including single nucleotide polymorphisms (SNPs) in genes related to tissue repair, protein folding, and inflammation.62,63 Epigenetic studies have found persistent molecular scars, altered DNA methylation patterns related to unresolved airway injury.64 These findings help distinguish between pathogenic processes that resolve (as in spontaneous remission) or persist (as in asthma). Combined with insights from immunomodulation trials,65,66 the growth in molecular information is clearing the path to transformative, curative therapies.
Building on decades of success and investment: the unique advantages of Australia
The CURE Asthma initiative is uniquely positioned to drive transformative progress by building on decades of leading clinical research, longitudinal cohort epidemiology, and fundamental basic and discovery research in Australia. These resources are the legacy of sustained national funding of asthma‐related research, including $348 million from the National Health and Medical Research Council for lung disease (2000–2022) and $283.8 million from the Medical Research Future Fund for respiratory health, although only a small fraction ($11.3 million; eight of 151 grants) was specifically for asthma research.17
A major strength of the CURE Asthma initiative is its access to eleven of the most valuable Australian longitudinal cohort studies, encompassing more than 75 000 participants for whom comprehensive phenotyping, archived biological samples, and longitudinal lung function data are available, coupled with the machine learning‐capable CURE–Asthma Data Integration Research Engine (CURE‐ADIRE). The included cohorts span more than 60 years across the entire lifespan, and the dataset includes family‐based data from parents, children, and siblings, as well as a limited amount of information for First Nations participants in some studies. The ongoing cohort studies are unparalleled in both scale and comprehensiveness, making it a national resource of international significance. We estimate that replicating these cohort studies would today cost more than $1 billion. For context and comparison, the three‐year international NOVELTY observational study of real world airway disease (12 000 participants, limited biological sample collection, and no lung function data, but including smaller sub‐studies)67 is estimated to have cost more than AU$160 million (NCT02760329)68 and the ten‐year United States COPDGene69 (genetic epidemiology of COPD) program (about 10 000 patients) has cost about AU$118 million (two National Institutes of Health U01 investigator‐initiated early stage clinical studies [2007–2011] and two R01 research project grants [2012–2016]).
By combining our exceptional national resources with cutting edge advances in molecular science, computational biology, and innovative methods, including digital twin technologies that more rapidly link discovery with clinical trials,50 the CURE Asthma initiative is an unprecedented opportunity for accelerating the translation of research findings and bringing curative therapies within reach. We are at the nexus of compelling unmet patient and medical need, technical feasibility, market appetite, and an advantageous national strategic position. We stand at the beginning of the next bioscience revolution and call on all who care about people with asthma — children and adults with mild to severe disease — to support our transformative vision.
The articles in this supplement outline current knowledge about the nature of asthma and a realistic research strategy for finding transformative new therapies. Taken together, they comprise the rationale and the roadmap supporting our call for large, sustained, and focused CURE Asthma funding over the coming decade.
Box 1 – Relative changes in the numbers of deaths attributed to asthma and acute myocardial infarction, Australia, 1990–2024*
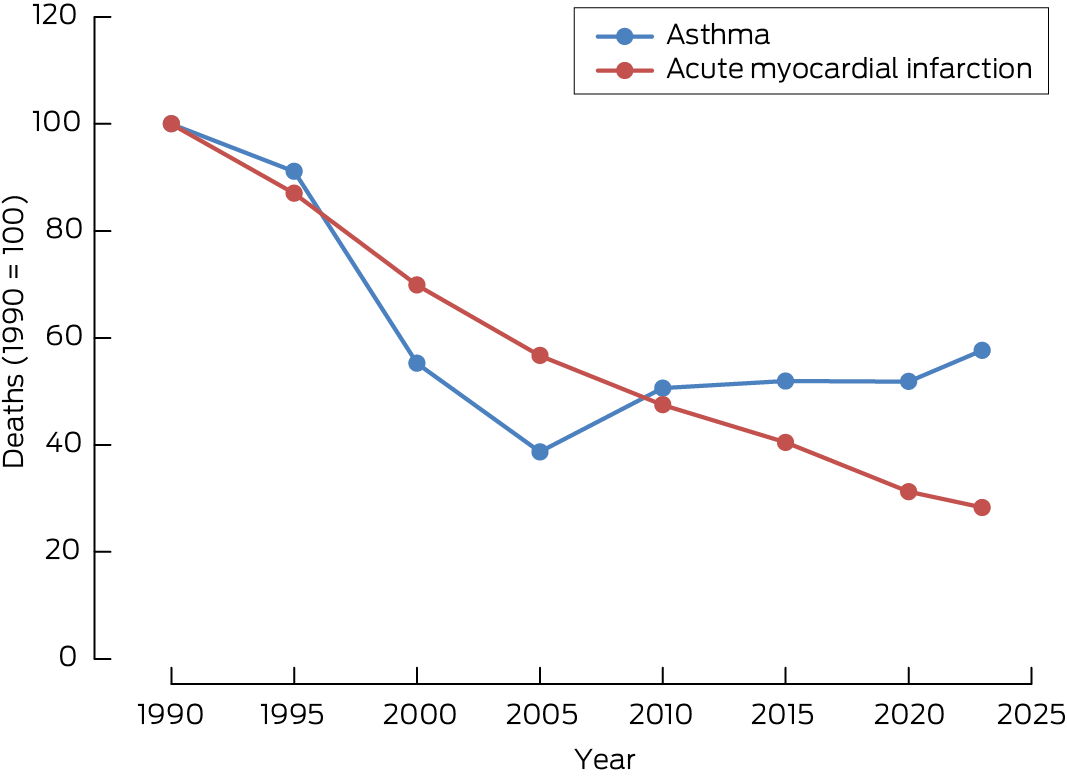
* Data source: Australian Bureau of Statistics (ref. 10 and corresponding archived data).
Box 2 – What CURE might mean for First Nations Australians*
|
|
|||||||||||||||
|
Before the arrival of the British colonisers, First Nations peoples participated in active lifestyles, accessed food sources high in nutrition and maintained sustainable ecosystems while caring for Country. The impact of colonisation, and ongoing settler colonialism, has contributed to the precipitous decline in Aboriginal and Torres Strait Islander health status over the last 230+ years. Historical, political, social, and economic factors, which frame both the past and present burden of disease, have contributed to substantial Indigenous health inequities. |
|||||||||||||||
|
Aboriginal and Torres Strait Islander peoples experience higher rates of chronic conditions such as diabetes, cardiovascular disease, and renal disease, which are avoidable conditions linked to the destruction of traditional cultures during colonisation, and contemporaneously, to the social determinants of Indigenous health, including history, settler colonialism, racism, intergenerational trauma, family, community, identity, and Country. These determinants are interwoven within broader mainstream social, political, and economic contexts in which First Nations people live. |
|||||||||||||||
|
Similarly, asthma is a serious condition experienced by First Nations peoples which often presents with other co‐morbidities and risk factors. Yet despite this, respiratory disease, including asthma, receives less attention than other chronic conditions, despite high hospitalisation and mortality rates. This unfair and remediable imbalance will be potentially redressed by the remediable research of CURE. |
|||||||||||||||
|
Research remains a contested space given the ongoing inequities and injustices in Aboriginal and Torres Strait Islander health. Historically, health research has often been undertaken without appropriate engagement, self‐determination, reciprocity, or respect, thus creating harm and little benefit for First Nations peoples. The research paradigm is gradually being transformed through privileging Aboriginal and Torres Strait Islander knowledge systems in collaborative approaches and with community ownership. |
|||||||||||||||
|
CURE has significant potential to alleviate asthma and close the gap for Aboriginal and Torres Strait Islander peoples for whom respiratory conditions may be extremely debilitating. The research team need to consider how they will meaningfully engage First Nations communities early in the CURE journey. This approach will include First Nations consumers and researchers, from the outset, in culturally appropriate ways to lead and embed Aboriginal and Torres Strait Islander perspectives. |
|||||||||||||||
|
It is critical for researchers to understand where, and for whom and how, asthma as a priority is positioned to facilitate meaningful engagement in research implementation, participation, and governance. Researchers should listen, learn, and evolve to become culturally safe and capable, in an environment where First Nations researchers may seek opportunities, and be leaders in the work. Non‐Indigenous researchers will learn how to use new knowledge and skills in cultural safety to minimise the impacts of unconscious bias and power differentials, and to engage in critical reflection on the influence of their values, attitudes and cultural beliefs when engaging in research. |
|||||||||||||||
|
The research program over the 10 years will not only adhere to the Australian Institute of Aboriginal and Torres Strait Islander Studies (AIATSIS) guidelines but will also include the engagement of First Peoples at all stages so the findings and translation will truly reflect First Peoples outcomes, priorities, and communication needs (nothing about us without us). |
|||||||||||||||
|
Aunty Wendy Edmondson, 2024. |
|||||||||||||||
|
CURE Asthma First Nations Knowledge Holder |
|||||||||||||||
|
|
|||||||||||||||
|
* In keeping with current cultural practice, the terms “Aboriginal and Torres Strait Islander”, “Indigenous”, and “First Nations” are used interchangeably in this text. This text was invited as an unreferenced narrative. Readers will find supporting information in references 23,24,25,26,27,28,29. |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- 1. Osler W. The principles and practice of medicine. New York: D Appleton & Company, 1892; p 295.
- 2. Global Initiative for Asthma. Frequently asked questions. Undated. https://ginasthma.org/about‐us/faqs (viewed Apr 2025).
- 3. Taylor R, Comino E, Bauman A. Asthma mortality in Australia 1920–94: age, period, and cohort effects. J Epidemiol Community Health 1997; 51: 408‐411.
- 4. Organisation for Economic Cooperation and Development. Health at a glance 2023. OECD indicators, 7 Nov 2023. https://www.oecd.org/en/publications/2023/11/health‐at‐a‐glance‐2023_e04f8239.html (viewed Apr 2025).
- 5. Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet 2018; 391: 350‐400.
- 6. Majellano EC, Bell RL, Flynn AW, et al. Identifying the asthma research priorities of people with asthma, their carers and other stakeholders. Respirology 2023; 28: 636‐648.
- 7. Brawley OW, Goldberg P. The 50 years’ war: the history and outcomes of the National Cancer Act of 1971. Cancer 2021; 127: 4534‐4540.
- 8. Enilari O, Sinha S. The global impact of asthma in adult populations. Ann Glob Health 2019; 85: 2.
- 9. Asher MI, Rutter CE, Bissell K, et al; Global Asthma Network Phase I Study Group. Worldwide trends in the burden of asthma symptoms in school‐aged children: Global Asthma Network Phase I cross‐sectional study. Lancet 2021; 398: 1569‐1580.
- 10. Australian Bureau of Statistics. Causes of death, Australia, 2023. 10 Oct 2024. https://www.abs.gov.au/statistics/health/causes‐death/causes‐death‐australia/latest‐release#data‐downloads (viewed Oct 2025).
- 11. Williams S, Sheikh A, Campbell H, et al; Global Health Respiratory Network. Respiratory research funding is inadequate, inequitable, and a missed opportunity [letter]. Lancet Respir Med 2020; 8: e67‐e68.
- 12. Pott H, Sykes DL, Charriot J, et al. Breathing barriers: bridging lung health, research, and awareness. Lancet Respir Med 2025; 13: 665‐667.
- 13. Mohan A, Lugogo NL, Hanania NA, et al. Questions in mild asthma: an official American Thoracic Society research statement. Am J Respir Crit Care Med 2023; 207: e77‐e96.
- 14. Foster JM, McDonald VM, Guo M, Reddel HK. “I have lost in every facet of my life”: the hidden burden of severe asthma. Eur Respir J 2017; 50: 1700765.
- 15. Asthma and Allergy Foundation of America. Asthma facts. Reviewed Apr 2025. https://aafa.org/asthma/asthma‐facts (viewed Oct 2025).
- 16. Asthma+Lung UK. Types of asthma. Reviewed 30 June 2024. https://www.asthmaandlung.org.uk/conditions/asthma/types‐asthma (viewed Oct 2025).
- 17. Australian Institute for Health and Welfare. Chronic respiratory conditions: asthma. Updated 27 Nov 2024. https://www.aihw.gov.au/reports/chronic‐respiratory‐conditions/asthma (viewed Apr 2025).
- 18. Australian Institute of Health and Welfare. Australia’s children: in brief (cat. no. CWS 72). 17 Dec 2019. https://www.aihw.gov.au/reports/children‐youth/australias‐children‐in‐brief/summary (viewed Oct 2025).
- 19. García‐Sanchez D, Darssan D, Lawler SP, et al. Asthma and anxiety development in Australian children and adolescents. Pediatr Allergy Immunol 2023; 34: e13941.
- 20. Hu N, Fardell J, Wakefield CE, et al School academic performance of children hospitalised with a chronic condition. Arch Dis Child 2022; 107: 289‐296.
- 21. Khan JR, Lingam R, Owens L, et al. Social deprivation and spatial clustering of childhood asthma in Australia. Glob Health Res Policy 2024; 9: 22.
- 22. Australian Institute for Health and Welfare. First Nations people with asthma. Updated 14 Dec 2023. https://www.aihw.gov.au/reports/chronic‐respiratory‐conditions/first‐nations‐people‐with‐asthma (viewed Apr 2025).
- 23. Anonymous. Caring for Country. In: Australia: state of the environment. 2021. https://soe.dcceew.gov.au/indigenous/management/caring‐country (viewed Sept 2025).
- 24. Victorian Public Sector Commission. Aboriginal and Torres Strait Islander culture and history. 28 June 2022. https://www.vpsc.vic.gov.au/workforce‐programs/aboriginal‐cultural‐capability‐toolkit/aboriginal‐culture‐and‐history (viewed Sept 2025).
- 25. Australian Department of Health, Disability and Ageing. Status and determinants of Aboriginal and Torres Strait Islander health. 30 June 2021. https://www.health.gov.au/topics/aboriginal‐and‐torres‐strait‐islander‐health/status‐and‐determinants (viewed Sept 2025).
- 26. Australian Department of Health, Disability and Ageing. Chronic disease support for Aboriginal and Torres Strait Islander people. Updated 21 Nov 2022. https://www.health.gov.au/topics/aboriginal‐and‐torres‐strait‐islander‐health/chronic‐disease‐support (viewed Sept 2025).
- 27. National Health and Medical Research Council. Ethical conduct in research with Aboriginal and Torres Strait Islander Peoples and communities. 2018. https://www.nhmrc.gov.au/about‐us/publications/ethical‐conduct‐research‐aboriginal‐and‐torres‐strait‐islander‐peoples‐and‐communities (viewed Sept 2025).
- 28. Morey K, Franks C, Pearson O, et al. Research ACCORDing to whom? Developing a South Australian Aboriginal and Torres Strait Islander Health Research Accord. First Nations Health and Wellbeing 2023; 1: 100003.
- 29. Australian Institute of Aboriginal and Torres Strait Islander Studies (AIATSIS). Ethical research. Updated 14 Aug 2025. https://aiatsis.gov.au/research/ethical‐research (viewed Sept 2025).
- 30. Katsaounou P, Odemyr M, Spranger O, et al. Still fighting for breath: a patient survey of the challenges and impact of severe asthma. ERJ Open Res 2018; 4: 00076‐2018.
- 31. Australian Bureau of Statistics. Asthma; 2022. 15 Dec 2023. https://www.abs.gov.au/statistics/health/health‐conditions‐and‐risks/asthma/latest‐release (viewed Apr 2025).
- 32. Scott KM, Von Korff M, Ormel J, et al. Mental disorders among adults with asthma: results from the World Mental Health Survey. Gen Hosp Psychiatry 2007; 29: 123‐133.
- 33. Australian Institute of Health and Welfare. Principal diagnosis data cubes. Separation statistics by principal diagnosis, 2020–21 to 2022–23. Updated: 8 July 2025. https://www.aihw.gov.au/reports/hospitals/principal‐diagnosis‐data‐cubes/contents/summary (viewed Oct 2025).
- 34. Emergency department care 2022–23. Updated 14 May 2025. https://www.aihw.gov.au/reports‐data/myhospitals/sectors/emergency‐department‐care (viewed Apr 2025).
- 35. Deloitte Access Economics. The hidden cost of asthma. Asthma Australia and National Asthma Council Australia. Nov 2015. https://asthma.org.au/wp‐content/uploads/2022/03/HIdden‐cost‐of‐asthma‐final‐report‐revised‐181115‐v2‐2.pdf (viewed Apr 2025).
- 36. Blakey J, Chung LP, McDonald VM, et al. Oral corticosteroids stewardship for asthma in adults and adolescents: a position paper from the Thoracic Society of Australia and New Zealand. Respirology 2021; 26: 1112‐1130.
- 37. Subbarao P. The final frontier: preschool asthma severity: the silent years no more. Ann Am Thorac Soc 2019; 16: 550‐552.
- 38. Cane R, Ranganathan S, McKenzie S. What do parents of wheezy children understand by “wheeze”? Arch Dis Child 2000; 82: 327‐332.
- 39. Barnes PJ, Pedersen S. Efficacy and safety of inhaled corticosteroids in asthma. Report of a workshop held in Eze, France, October 1992. Am Rev Respir Dis 1993; 148: S1‐S26.
- 40. Reddel H, Ampon R, Davis S, et al. Asthma outcomes in Australia: changes from 2012 to 2021 [abstract: Australia and New Zealand Society of Respiratory Science/Thoracic Society of Australia and New Zealand annual scientific meeting for leaders in lung health and respiratory science, Christchurch, New Zealand, 25–28 March 2023]. Respirology 2023; 28 (Suppl 2): 126.
- 41. Hew M, McDonald VM, Bardin PG, et al. Cumulative dispensing of high oral corticosteroid doses for treating asthma in Australia. Med J Aust 2020; 213: 316‐320. https://www.mja.com.au/journal/2020/213/7/cumulative‐dispensing‐high‐oral‐corticosteroid‐doses‐treating‐asthma‐australia
- 42. Pandey D. Asthma drugs market size, share, and trends 2025 to 2034. Precedence Research, updated 22 Jan 2025. https://www.precedenceresearch.com/asthma‐drugs‐market (viewed Apr 2025).
- 43. Middleton PG, Mall MA, Dřevínek P, et al; VX17‐445‐102 Study Group. Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 2019; 381: 1809‐1819.
- 44. IndustryARC. Cystic fibrosis therapeutics market: by drug class, by route of administration, by age group, by distribution channel, and by geography. Opportunity analysis and industry forecast, 2025–2031. 1 Oct 2025. https://www.industryarc.com/Research/cystic‐fibrosis‐therapeutics‐market‐801250 (viewed Oct 2025).
- 45. Husain M, Birkenfeld AL, Donsmark M, et al; PIONEER 6 Investigators. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019; 381: 841‐851.
- 46. Dunleavy K. Novo, Lilly set to dominate $71B GLP‐1 drug market by 2032: J.P. Morgan. FiercePharma, 11 Sept 2023. https://www.fiercepharma.com/pharma/after‐promising‐heart‐data‐novo‐nordisks‐wegovy‐jp‐morgan‐doubles‐2032‐market‐projection‐71b (viewed Oct 2025).
- 47. Shiravand Y, Khodadadi F, Kashani SMA, et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol 2022; 29: 3044‐3060.
- 48. Verified Market Research. Immune checkpoint inhibitors market size, share, trends and forecast. Oct 2024. https://www.verifiedmarketresearch.com/product/immune‐checkpoint‐inhibitors‐market (viewed Oct 2025).
- 49. Spencer D. What effect have weight loss drugs had on the Scandinavian economy? DDW‐Online, 27 Feb 2025. https://www.ddw‐online.com/what‐effect‐have‐weight‐loss‐drugs‐had‐on‐the‐scandinavian‐economy‐33689‐202502 (viewed Oct 2025).
- 50. Bourduka M, Arneth AJ, Marakov N, et al. Generative AI and digital twins: shaping a paradigm shift from precision to truly personalized medicine. Expert Opin Drug Discov 2025; 20: 821‐826.
- 51. Kohn DB, Booth C, Shaw KL, et al. Autologous ex vivo lentiviral gene therapy for adenosine deaminase deficiency. N Engl J Med 2021; 384: 2002‐2013.
- 52. Locatelli F, Lang P, Wall D, et al; CLIMB THAL‐111 Study Group. Exagamglogene autotemcel for transfusion‐dependent beta‐thalassemia. N Engl J Med 2024; 390: 1663‐1676.
- 53. Mullard A. FDA approves l‐amino acid decarboxylase deficiency gene therapy [news]. Nat Rev Drug Discov 2025; 24: 6.
- 54. Thomas D, McDonald VM, Pavord ID, Gibson PG. Asthma remission: what is it and how can it be achieved? Eur Respir J 2022; 60: 2102583.
- 55. Thomas D, McDonald VM, Gibson PG, Kim R. Defining “cure” for the asthmas. Med J Aust 2025; 223 (10 Suppl): 000‐000.
- 56. Pangalos MN. LinkedIn: About. 2025. https://www.linkedin.com/in/mene‐pangalos/?originalSubdomain=uk (viewed Apr 2025).
- 57. Vonk J, Postma D, Boezen H, et al. Childhood factors associated with asthma remission after 30 year follow up. Thorax 2004; 59: 925‐929.
- 58. Thomas D, McDonald VM, Stevens S, et al. Biologics (mepolizumab and omalizumab) induced remission in severe asthma patients. Allergy 2024; 79: 384‐392.
- 59. Upham JW, Le Lievre C, Jackson DJ, et al. Defining a severe asthma super‐responder: findings from a Delphi process. J Allergy Clin Immunol Pract 2021; 9: 3997‐4004.
- 60. Thomas D, McDonald VM, Stevens S, et al. Effect of azithromycin on asthma remission in adults with persistent uncontrolled asthma: a secondary analysis of a randomized, double‐anonymized, placebo‐controlled trial. Chest 2024; 166: 262‐270.
- 61. Shackleford A, Heaney LG, Redmond C, et al. Clinical remission attainment, definitions, and correlates among patients with severe asthma treated with biologics: a systematic review and meta‐analysis. Lancet Respir Med 2025; 13: 23‐34.
- 62. Sayers I, John C, Chen J, Hall IP. Genetics of chronic respiratory disease. Nat Rev Genet 2024; 25: 534‐547.
- 63. Herrera‐Luis E, Martin‐Almeida M, Pino‐Yanes M. Asthma: genomic advances toward risk prediction. Clin Chest Med 2024; 45: 599‐610.
- 64. Vermeulen CJ, Xu CJ, Vonk JM, et al. Differential DNA methylation in bronchial biopsies between persistent asthma and asthma in remission. Eur Respir J 2020; 55: 1901280.
- 65. Woehlk C, Ramu S, Sverrild A, et al. Allergen immunotherapy enhances airway epithelial antiviral immunity in patients with allergic asthma (VITAL study): a double‐blind randomized controlled trial. Am J Respir Crit Care Med 2023; 207: 1161‐1170.
- 66. Koatz AM, Coe NA, Cicerán A, Alter AJ. Clinical and immunological benefits of OM‐85 bacterial lysate in patients with allergic rhinitis, asthma, and COPD and recurrent respiratory infections. Lung 2016; 194: 687‐697.
- 67. Reddel HK, Gerhardsson de Verdier M, Agustí A, et al. Prospective observational study in patients with obstructive lung disease: NOVELTY design. ERJ Open Res 2019; 5: 00036‐2018.
- 68. AstraZeneca. NOVELTY: a longitudinal observational study of asthma and COPD [ClinicalTrials.gov NCT02760329]. Updated 3 Oct 2024. https://clinicaltrials.gov/study/NCT02760329 (viewed Oct 2025).
- 69. Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010; 7: 32‐43.





The University of Melbourne, Curtin University, the University of Western Australia, the Institute for Respiratory Health, the George Institute for Global Health, Asthma Australia, and the University of Newcastle provided the workspace, infrastructure and intellectual environment to enable its staff to contribute to the CURE Asthma Initiative supplement. Vanessa McDonald and Christine Jenkins have received National Health and Medical Research Council and Medical Research Future Fund research funding, and Vanessa McDonald receives funding from GSK for a research project, all unrelated to this article. The graph in Box 1 was prepared by Zoe Castillo (Institute for Respiratory Health, Western Australia).
Vanessa McDonald has received education honoraria and travel costs from Boehringer Ingelheim, GSK, and the Menarini Foundation. John Blakey has received consulting fees from GSK, AstraZeneca, Sanofi and Chiesi. Gary Anderson has received speaker honoraria from AstraZeneca and GSK, and consultancy payments in past five years from DevPro, ENA Respiratory, and Pieris Pharmaceutical. Gary Anderson is developing a molecule that could be patented for use in asthma therapy; no royalties have been received to date.
Author contributions:
All authors: conceptualisation, writing (review and editing). Anthony Flynn, John Blakey, Gary Anderson: funding acquisition. Anthony Flynn, Gary Anderson: project administration, writing (original draft).